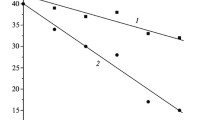
X-ray electron spectroscopy and thermodynamic analysis are used to investigate the sequence of chemical transformations of the elements of a surface layer of silicate glass in the system PbO – BaO – Na2O – Al2O3 – SiO2 under heating in a hydrogen atmosphere. The process of thermal reduction of a multicomponent lead-silicate glass in hydrogen is a multistage process of diffusion and chemical interaction of lead with hydrogen. Reduction of lead is observed, the lead atoms bound with aluminum-oxygen groupings interacting being the first to interact with hydrogen after which the lead atoms in the silicon-oxygen structural units interact with hydrogen. The remaining components of the glass do not interact with hydrogen in the temperature range 375–475°C. The concentration profiles of the elemental distribution in the surface layer of the reduced glasses are determined.
Similar content being viewed by others
References
V. I. Beloglazov, S. P. Sukhoveev, and N. V. Suetin, “Creation of micron and submicron three-dimensional structures using glass-fiber technology,” Nano- and Mikrosistem. Tekh., No. 1, 6–9 (2000).
A. M. Tyutikov, “On the regime for reduction of certain lead-silicate glasses used for fabricating microchannel plates,” Optikomekh. Prom-st., No. 9, 41–45 (1974).
N. A. Vatolin, G. K. Moiseev, and B. G. Trusov, Thermodynamic Modeling in High-Temperature Inorganic Systems [in Russian], Metallurgiya, Moscow (1994).
O. M. Kanunnikova and O. Yu. Goncharov, “Interaction of leadsilicate glasses with hydrogenonheating. 1: Chemical transformations in the system PbO – SiO2 – H2,” Fiz. Khim. Obrab. Mater., No. 2, 74–77 (2006).
L. V. Gurvich, I. V. Veits, V. A. Medvedev, et al., Handbook of the Thermodynamic Properties of Individual Substances [in Russian], Nauka, Moscow (1982), Vols. 1–4.
O. M. Kanunnikova, F. Z. Gil’mutdinov, V. I. Kazhevnikov, and M. F. Sorokina, “Thermally stimulated segregation of elements in the surface layer of lead-silicate glasses,” Fiz. Khim Obrab. Mater., No. 4, 70–73 (1996).
F. Z. Gil’mutdinov and O. M. Kanunnikova, “Prediction of the change of the surface composition of multicomponent alloys under thermal actions,” Fiz. Met. Metalloved., 84(2), 78–88 (1997).
N. A. Toropov, V. P. Barzakovskii, V. V. Lapin, and N. N. Kurtseva, Handbook of Phase Diagrams of Silicate Systems [in Russian], Nauka, Leningrad (1969), Vol. 1.
Author information
Authors and Affiliations
Additional information
Translated from Steklo i Keramika, No. 2, pp. 12–14, February, 2009.
Rights and permissions
About this article
Cite this article
Kanunnikova, O.M., Goncharov, O.Y. Formation of a surface layer of multicomponent lead-silicate glasses in hydrogen on heating. Glass Ceram 66, 53–56 (2009). https://doi.org/10.1007/s10717-009-9122-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-009-9122-x