Abstract
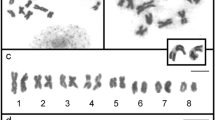
Fluorescence in situ hybridization (FISH) using telomeric and ribosomal sequences was performed in four species of toad genus Chaunus: C. ictericus, C. jimi, C. rubescens and C. schneideri. Analyses based on conventional, C-banding and Ag-NOR staining were also carried out. The four species present a 2n = 22 karyotype, composed by metacentric and submetacentric chromosomes, which were indistinguishable either after conventional staining or banding techniques. Constitutive heterochromatin was predominantly located at pericentromeric regions, and telomeric sequences (TTAGGG)n were restricted to the end of all chromosomes. Silver staining revealed Ag-NORs located at the short arm of pair 7, and heteromorphism in size of NOR signals was also observed. By contrast, FISH with ribosomal probes clearly demonstrated absence of any heteromorphism in size of rDNA sequences, suggesting that the difference observed after Ag-staining should be attributed to differences in chromosomal condensation and/or gene activity rather than to the number of ribosomal cistrons.







Similar content being viewed by others
Abbreviations
- ITS:
-
Interstitial telomeric sites
- FISH:
-
Fluorescence in situ hybridization
- FN:
-
Fundamental number
- NORs:
-
Nucleolar organizer regions
References
Amaro-Ghilardi RC, Rodrigues MT, Yonenaga-Yassuda Y (2004) Chromosomal studies after differential staining and fluorescence in situ hybridization using telomeric probe in three Leptodactylus species. Caryologia 57:53–65
Azevedo MFC, Foresti F, Ramos PRR, Jim J (2003) Comparative cytogenetic studies of Bufo ictericus, B. paracnemis (Amphibia, Anura) and a intermediate form in sympatry. Genet Mol Biol 26:289–294
Baldissera FA, Batistic RF, Haddad CFB (1999) Cytotaxonomic considerations with the descripition of two new NOR locations for South American toads, genus Bufo (Anura: Bufonidae). Amphib-reptil 20:413–420
Beçak ML (1968) Chromosomal analysis of eighteen species of Anura. Caryologia 21:191–208
Beckert WH, Doyle BW (1968) Bufo regularis, a twenty—hromosome toad. Genet Res 11:209–210
Bogart JP (1968) Chromosome number difference in the amphibian genus Bufo: the Bufo regularis species group. Evolution 22:42–45
Bogart JP (1972) Karyotypes. In: Blair WF (ed) Evolution in the genus Bufo. Univ. Texas Press, Austin, pp 171–195
Bogart JP (1973) Method for obtaining chromosomes. Caldasia XI:29–40
Bogart JP, Perret JP (1977) The karyotype of Bufo danielae Perret. Rev Suisse Zool 84:501–504
Bogart JP, Tandy M (1976) Polyploid amphibians: three more diploid–etraploid cryptic species of frogs. Science 193:334–335
Brum-Zorilla N, Saez FA (1971) Chromosome of South American Bufonidae (Amphibia anura). Experientia 27:470–471
Fagundes V, Vianna-Morgante AM, Yonenaga-Yassuda Y (1997) Telomeric sequences localization and G-banding patterns in the identification of a polymorphic chromosomal rearrangement in the rodent Akodon cursor (2n = 14, 15 and 16). Chrom Res 5:228–232
Fagundes V, Scalzi-Martin JM, Sims K, Hozier J, Yonenaga-Yassuda Y (1997) Zoo-FISH of a microdissection DNA library and G-banding patterns reveal the homeology between the Brazilian rodents Akodon cursor and A. montensis. Cytogenet Cell Genet 78:224–228
Foote DL, Wiley JE, Little ML, Meyne J (1991) Ribosomal RNA gene site polymorphism in Bufo terrestris. Cytogenet Cell Genet 57:196–199
Formas JR (1978) The chromosomes of Bufo rubropunctatus and Bufo chilensis (Anura, Bufonidae) and other species of the spinulosus group. Experientia 34:452–454
Frost DR (2007) Amphibian species of the world: an online reference. Version 5.0 (1 February, 2007). Electronic database accessible at http://research.amnh.org/herpetology/amphibia/index.php. American Museum of Natural History, New York, USA
Frost DR, Grant T, Faivovich J, Bain RH, Haas A, Haddad CFB, De Sá RO, Channing A, Wilkinson M, Donnelan SC, Raxworthy CJ, Campbell JA, Blotto BL, Moler P, Drewes RC, Nussbaum RA, Lynch JD, Green DM, Wheeler WC (2006) The amphibian tree of life. Bull Am Mus Nat Hyst 297:1–370
Fujiwara A, Abe S, Yamaha E, Yamazaki F, Yoshida MC (1998) Chromosomal localization and heterochromatin association of ribosomal RNA gene loci and silver-stained nucleolar organizer regions in salmonid fishes. Chrom Res 6:463–471
Garagna S, Ronchetti E, Mascheretti S, Crovella S, Formenti D, Rumpler Y, Romanini M (1997) Non-telomeric chromosome localization of (TTAGGG)n repeats in the genus Eulemur. Chrom Res 5:487–491
Howell WM, Black DA (1980) Controlled silver staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia 36:1014–1015
Kasahara S, Silva APZ, Haddad CFB (1996) Chromosome banding in three species of Brazilian toads (Amphibia-Bufonidae). Braz J Genet 19:237–242
King M (1990) Animal cytogenetics. In: Jonh B, Gwent C (eds) Chordata 2. Amphibia, vol 4. Gebrüeder Borntraeger, Berlim. 241 p
Kuramoto M (1990) A list of chromosome numbers of anuran amphibians. Bull Fukuoka Univ Educ 39:83–127
Lee C, Sasi R, Lin CC (1993) Interstitial localization of telomeric DNA sequences in the Indian muntjac chromosomes: further evidence for tandem chromosome fusions in the karyotypic evolution of the Asian muntjacs. Cytogenet Cell Genet 63:156–159
Lourenço LB, Recco-Pimentel S, Cardoso AJ (1998) Polymorphism of the nucleolus organizer regions (NORs) in Physalaemus petersi (Amphibia, Anura, Leptodactylidae) detected by silver staining and fluorescence in situ hybridization. Chrom Res 6:621–628
Matsui M, Seto T, Kohsaka Y, Borkin LJ (1985) Bearing of chromosome C-banding patterns on the classification of eurasian toads of the Bufo bufo complex. Amphib-reptil 6:23–33
Meyne J, Ratliff RL, Moysis RK (1989) Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci USA 86:7049–7053
Meyne J, Baker RJ, Hobart HH, Hsu TC, Ryder OA, Ward OA, Wiley JE, Wurster-Hill DH, Yates TL, Moysis RK (1990) Distribution of non-telomeric sites of the (TTAGGG)n telomeric sequence in vertebrate chromosomes. Chromosoma 99:3–10
Miura I (1995) Two differentiated groups of the Japanese toad, Bufo japonicus japonicus, demonstrated by C-banding analysis of chromosomes. Caryologia 48:123–136
Morescalchi A, Gargiulo G (1968) Su alcune relazioni cariologiche del genero Bufo (Amphibia, Salientia). Rend Acc Sci Fis Mat S4 35:117–120
Piazze MER (1995) Cariotipo y patrones de bandas C en Bufo spinulosus arequipensis (Amphibia: Anura). Rev Ecol Lat Am 2:5–11
Porter CA, Hamilton MJ, Sites JW, Baker RJ (1991) Location of ribosomal DNA in chromosomes of squamate reptiles: systematic and evolutionary implications. Herpetologica 47:271–280
Pramuk JB (2006) Phylogeny of South American Bufo (Anura: Bufonidae) inferred from combined evidence. Zool J Linn Soc 146:407–452
Qumsiyeh MB, Coate JL, Peppers JA, Kennedy PK, Kennedy ML (1997) Robertsonian chromosomal rearrangements in short-tailed shrew, Blarina carolinensis, in western Tennessee. Cytogenet Cell Genet 76:153–158
Scherthan H (1990) Localization of the repetitive telomeric sequence (TTAGGG)n in two muntjac species and implications for their karyotypic evolution. Cytogenet Cell Genet 53:115–117
Schmid M (1978) Chromosome banding in Amphibia I: constitutive heterochromatin and nucleolus organizer regions in Bufo and Hyla. Chromosoma 66:361–388
Schmid M (1980) Chromosome banding in Amphibia IV. Differentiation of CG- and AT-rich chromosome regions in Anura. Chromosoma 77:83–103
Schmid M (1982) Chromosome banding in Amphibia VII: analysis of the structure and variability of NORs in Anura. Chromosoma 87:327–344
Schmid M, Feichtinger W, Nanda I, Schakowski R, Garcia RV, Puppo JM, Badillo AF (1994) An extraordinary low diploid chromosome number in the reptile Gonatodes taniae (Squamata, Gekkonidae). J Hered 85:255–260
Schmid M, Feichtinger W, Steinlein C, Rupprecht A, Haaf T, Kaiser H (2002a) Chromosome banding in Amphibia XXIII. Giant W sex chromosomes and extremely small genomes in Eleutherodactylus euphronides and Eleutherodactylus shrevei (Anura, Leptodactylidae). Cytogenet Genome Res 97:81–94
Schmid M, Ziegler CG, Steinlein C, Nanda I, Haaf T (2002b) Chromosome banding in Amphibia XXIV. The B chromosome of Gastrotheca espeletia (Anura, Hylidae). Cytogenet Genome Res 97:205–218
Schmid M, Feichtinger W, Steinlein C, Haaf T, Schartl M, Garcia RV, Puppo JM, Badillo AF (2002c) Chromosome banding in Amphibia XXVI. Coexistence of homomorphic XY sex chromosomes and a derived Y-autossome translocation in Eleutherodactylus maussi (Anura, Leptodactylidae). Cytogenet Genome Res 99:330–343
Schmid M, Feichtinger W, Steinlein C, Garcia RV, Badillo AF (2003) Chromosome banding in Amphibia XXVIII. Homomorphic XY sex chromosomes and a derived Y-autossome translocation in Eleutherodactylus riveroi (Anura, Leptodactylidae). Cytogenet Genome Res 101:62–73
Silva MJJ, Yonenaga-Yassuda Y (1997) New karyotypes of two related species of Oligoryzomys genus (Cricetidae, Rodentia) involving centric fusion with loss of NORs and distribution of telomeric (TTAGGG)n sequences. Hereditas 127:217–229
Silva MJJ, Yonenaga-Yassuda Y (1998a) Karyotype and chromosomal polymorphism of an undescribed Akodon from Central Brazil, a species with the lowest known diploid chromosome number in rodents. Cytogenet Cell Genet 81:46–50
Silva MJJ, Yonenaga-Yassuda Y (1998b) Heterogeneity and meiotic behaviour of B and sex chromosomes, banding patterns and localization of (TTAGGG)n sequences by fluorescence in situ hybridization in the neotropical water rat Nectomys (Rodentia, Cricetidae). Chrom Res 6:455–462
Sumner AT (1972) A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res 75:304–306
Svartman M, Vianna-Morgante AM (1998) Karyotype evolution of marsupials: from higher to lower diploid numbers. Cytogenet Cell Genet 82:263–266
Vermeesch JR, De Meurichy W, Van Den Berghe H, Marynen P, Petit P (1996) Differences in the distribution and nature of the interstitial telomeric (TTAGGG)n sequences in the chromosomes of the Giraffidae, okapi (Okapia johnstoni), and giraffe (Giraffa camelopardalis): evidence for ancestral telomeres at the okapi polymorphic rob(4;26) fusion site. Cytogenet Cell Genet 72:310–315
Wiley JE, Meyne J, Little ML, Stout JC (1992) Interstitial hybridization sites of the (TTAGGG)n telomeric sequence on the chromosomes of some North American hylid frogs. Cytogenet Cell Genet 61:55–57
Acknowledgments
The authors are grateful to Alexandre Uarth Christoff, Juliana Marge Pagnozzi, Pedro Luis Bernardo da Rocha, Raquel Pena de Oliveira and Valéria Fagundes for collecting the specimens, and to Tien Hsi Chu and Miriam Romeo for technical assistance. We are very grateful to Katia Cristina Machado Pellegrino for the valuable comments and suggestions on the manuscript. Grants for this study were provided by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). The specimens were collected under permission of Instituto Brasileiro do Meio Ambiente e Recursos Naturais Renováveis (IBAMA no 02001.003451/93-13 AC and no 02001.002750/96-93).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amaro-Ghilardi, R.C., Silva, M.J.d.J., Rodrigues, M.T. et al. Chromosomal studies in four species of genus Chaunus (Bufonidae, Anura): localization of telomeric and ribosomal sequences after fluorescence in situ hybridization (FISH). Genetica 134, 159–168 (2008). https://doi.org/10.1007/s10709-007-9218-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-007-9218-6