Abstract
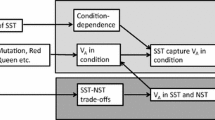
In theory, females of many species choose mates based on traits that are indicators of male genetic quality. A fundamental question in evolutionary biology is why genetic variation for such indicator traits persists despite strong persistent selection imposed by female preference, which is known as the lek paradox. One potential solution to the lek paradox suggests that the traits that are targets of mate choice should evolve condition-dependent expression and that condition should have a large genetic variance. Condition is expected to exhibit high genetic variance because it is affected by a large number of physiological processes and hence, condition-dependent traits should ‘capture’ variation contributed by a large number of loci. We suggest that a potentially important cause of variation in condition is competition for limited resources. Here, we discuss a pair of models to analyze the evolutionary genetics of traits affected by success in social competition for resources. We show that competition can contribute to genetic variation of ‘competition-dependent’ traits that have fundamentally different evolutionary properties than other sources of variation. Competition dependence can make traits honest indicators of genetic quality by revealing the relative competitive ability of males, can provide a component of heritable variation that does not contribute to trait evolution, and can help maintain heritable variation under directional selection. Here we provide a general introduction to the concept of competition dependence and briefly introduce two models to demonstrate the potential evolutionary consequences of competition-dependent trait expression.


Similar content being viewed by others
References
Adell JC, Molina V, Castro JA et al (1989) Unmasking frequency-dependent selection in tri-cultures of Drosophila melanogaster. Genetica 79:77–83
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Asmussen MA, Basnayake E (1990) Frequency-dependent selection: the high potential for permanent genetic variation in the diallelic, pairwise interaction model. Genetics 125:215–230
Bijma P, Muir WM, Ellen E et al (2007) Multilevel selection 2: estimating the genetic parameters determining inheritance and response selection. Genetics 175:289–299
Borgia G (1979) Sexual selection and the evolution of mating systems. In: Blum MS, Blum MN (eds) Sexual selection and reproductive competition in the insects. Academic Press, London, pp 19–80
Bürger R (2000) The mathematical theory of selection, recombination, and mutation. John Wiley & Sons, Chichester, UK
Bürger R, Gimelfarb A (2004) The effects of intraspecific comeptition and stabilizing selection on a polygenic trait. Genetics 167:1425–1443
Castro JA, Moya A, Mènsua JL (1985) Comeptitive selection in mono-, di- and tri-genotype cultures of Drosophila melanogaster. Zeitschrift Zool System Evol 23:214–228
Colegrave N (1993) Does larval competition affect fecundity independently of its effect on adult weight? Ecol Entomol 18:275–277
Dickerson GE (1955) Genetic slippage in response to selection for multiple objectives. Cold Spring Harb Symp Quant Biol 20:213–224
Fisher RA (1930) The genetical theory of natural selection. Clarendon Press, Oxford
Frank SA, Slatkin M (1992) Fisher’s fundamental theorum of natural selection. Trends Ecol Evol 7:92–95
Hamilton WD, Zuk M (1982) Heritable true fitness and bright birds: a role for parasites?. Science 218:384–387
Harris WE, McKane AJ, and Wolf JB (in press) The maintenance of heritable variation through social competition. Evolution
Hemmat M, Eggleston P (1989) Analysis of competitive interactions in tricultures of Drosophila melanogaster. Heredity 64:215–222
Höglund J, Alatalo RV (1995) Leks. Princeton University Press, Princeton
Iwasa Y, Pomiankowski A (1994) The evolution of mate preferences for multiple sexual ornaments. Evolution 48:853–867
Iwasa Y, Pomiankowski A, Nee S (1991) The evolution of costly mate preferences. II. The “handicap” principle. Evolution 45:1431–1442
Jennions MD, Petrie M (1997) Variation in mate choice and mating preferences: a review of causes and consequences. Biol Rev (Cambridge) 72:283–327
Kirkpatrick M, Ryan MJ (1991) The evolution of mating preferences and the paradox of the lek. Nature 350:33–38
Kotiaho JS, Simmons LW, Tomkins JL (2001) Towards a resolution of the lek paradox. Nature 410:684–686
Maynard Smith J (1982) Evolution and the theory of games. Cambridge University Press, Cambridge
de Miranda JR, Hemmat M, Eggleston P (1991) The competition diallel and the exploitation and interference components of larval competition in Drosophila melanogaster. Heredity 66:333–342
Moore AJ, Brodie ED III, Wolf JB (1997) Interacting phenotypes and the evolutionary process: I. direct and indirect genetic effects of social interactions. Evolution 51:1352–1362
Mousseau TA, Sinervo B, Endler JA (2000) Adaptive genetic variation in the wild. Oxford University Press, New York
Mueller LD (1988) Evolution of competitive ability in Drosophila by density-dependent natural selection. Proc Natl Acad Sci U S A 85:4383–4286
Muir WM (2005) Incorporation of competitive effects in forest tree or animal breeding programs. Genetics 170:1247–1259
Partridge L, Endler JA (1987) Life history constraints on sexual selection. In: Bradbury JW, Andersson M (eds) Sexual selection: testing the alternatives. John Wiley & Sons Ltd, pp 265–277
Pèrez-Tomè JM, Toro MA (1982) Competition of similar and non-similar genotypes. Nature 299:153–154
Pomiankowski A, Møller AP (1995) A resolution of the lek paradox. Proc R Soc B Biol Sci. 260:21–29
Rowe L, Houle D (1996) The lek paradox and the capture of genetic variation by condition dependent traits. Proc R Soc B Biol Sci 263:1415–1421
Sinervo B, Lively CM (1996) The rock-paper-scissors game and the evolution of alternative male strategies. Nature 380:240–243
Tomkins JL, Radawan J, Kotiaho JS et al (2004) Genic capture and resolving the lek paradox. Trends Ecol Evol 19:323–328
Weisbrot DR (1966) Genotypic interactions among competing strains and species of Drosophila. Genetics 53:422–435
Wolf J (2003) Genetic architecture and evolutionary constraint when the environment contains genes. Proc Natl Acad Sci U S A 100:4655–4660
Wolf JB, Brodie ED III, Cheverud JM et al (1998) Evolutionary consequences of indirect genetic effects. Trends Ecol Evol 13:64–69
Acknowledgements
We thank Allen Moore, Susan Riechert, Joshua Mutic and Richard Preziosi for discussions that have influenced our work on this topic. This work was supported by grants from the National Science Foundation (USA), the Natural Environment Research Council (UK) and the Biotechnology and Biological Sciences Research Council (UK).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wolf, J.B., Harris, W.E. & Royle, N.J. The capture of heritable variation for genetic quality through social competition. Genetica 134, 89–97 (2008). https://doi.org/10.1007/s10709-007-9214-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-007-9214-x