Abstract
Phosphorus (P) crop fertilization requires optimal management to avoid the waste of a non-renewable resource and water pollution, but current methods for assessing soil phyto-available P and plant P requirements are not sufficiently precise to meet this goal. The objectives of the present study were to (1) evaluate the effect of long-term P fertilization on the grain yield of winter wheat, maize, and rapeseed, (2) validate or establish models of critical shoot P concentration (PC) based on relationships of shoot P concentration with either shoot biomass or shoot nitrogen (N) concentration, and (3) assess both plant-based and soil-based diagnostic tools for managing P fertilization. A long-term field experiment with contrasted P fertilizer treatments, established in 1971 by Agroscope in Changins (Switzerland), was used to measure the shoot biomass and P concentration of winter wheat in 2011, maize in 2012, and rapeseed in 2014 weekly during the growing period and the grain yield at harvest. Soil available P in the 0–0.20 m soil layer was assessed by three chemical extractions: ammonium acetate EDTA (P-AAE), sodium bicarbonate (P-NaHCO3), and CO2-saturated water (P-CO2). Long-term P fertilization increased soil available P extracted by P-CO2 (+ 24%), P-AAE (+ 200%), and P-NaHCO3 (+ 155%), shoot growth and grain yield by 8.4% and 26.2% for winter wheat and rapeseed respectively but had no effect on maize. The relationships between PC and shoot biomass or N concentration were described respectively by allometric and linear models (R2 > 0.85, n = 21, 28 and 32 for winter wheat, maize and rapeseed respectively; slope P values for linear models < 0.05). The PC–shoot N concentration model (slope: 0.083, intercept: 0.88) for winter wheat confirmed results from previous studies and can be used for calculating the P nutrition index. For the three soil available P indicators, threshold values needed to achieve 95% of the maximum yield for the three crops were less than those currently used in the official fertilization guidelines in Switzerland. Our results obtained after 44 years of contrasted P fertilization confirm the relationship between PC and shoot N concentration for grain crops and the need to revise P fertilizer recommendations based on currently used soil P tests.
Similar content being viewed by others
Introduction
Phosphorus (P) is an essential crop nutrient (Marschner 1995) and its application to agricultural soils is crucial to achieve optimum crop production. Phosphorus fertilizer applied in excess to crop requirements, however, is an important environmental concern because soil P accumulation increases the risk of P losses to surface and ground waters, with detrimental effects on aquatic ecosystems through eutrophication (Haygarth and Jarvis 1999; Carpenter 2005). In addition, reserves of economically exploitable phosphate rocks are decreasing and some scenarios, even if controversial, predict its depletion in the next 100 years (Gilbert 2009). Therefore, for economic and environmental reasons, appropriate diagnostic systems and management practices are required to improve soil and fertilizer P use efficiency by crops.
Fertilization recommendations are generally based on soil P analyses that approximate the amount of available P to plants using chemical extractions (Demaria et al. 2005). The degree of success of soil chemical extractions in assessing plant-available P and predicting the amount of P fertilizer required to reach optimal crop yields is often limited. The absence of a yield response to P fertilization in fields with an expected positive response based on soil P analyses was reported for perennial forage grasses (Bélanger and Ziadi 2008; Messiga et al. 2015), wheat (Valkama et al. 2011; Bélanger et al. 2015b), maize (Ziadi et al. 2014) and rapeseed (McKenzie et al. 2003; Grant et al. 2009; Bélanger et al. 2015a). Those studies, however, were conducted over only a few years and the effects of long-term P fertilization on soil available P and grain yield were not investigated.
Plant-based diagnostic methods can be used as an alternative to or alongside soil analyses to evaluate crop P requirements. The critical P concentration (PC), defined as the minimal plant P concentration for maximal yield, is necessary for determining the crop P status and managing P fertilization for optimal plant nutrition. Most plant nutrient concentrations, such as N, P and K, decrease with increasing shoot biomass and plant development. Consequently, the P concentration in itself has no diagnostic value unless it is interpreted for a given level of shoot biomass. To account for this dilution effect, models of critical concentrations were established based on the relationship between the nutrient concentration and shoot dry matter (DM) yield under non-limiting conditions. This approach was first developed for N in grasslands (Salette and Lemaire 1981), and then tested for winter wheat (Greenwood et al. 1990; Justes et al. 1994), spring wheat (Ziadi et al. 2010), and maize (Ziadi et al. 2008b); it was then also used for P in various crops, including spring wheat (Bélanger et al. 2015a) and forage grasses (Bélanger and Ziadi 2008).
As a result of the dilution of both nutrients with increasing shoot biomass, P and N concentrations are positively related. This relationship has been used to develop models of PC expressed as a function of N concentration for spring wheat (Ziadi et al. 2008a; Bélanger et al. 2015b), maize (Ziadi et al. 2007), rapeseed (Bélanger et al. 2015a), and forage grasses (Bélanger and Ziadi 2008; Bélanger et al. 2017). The PC models can then be used to evaluate the plant P nutrition status via the P nutrition index (PNI), calculated as the ratio between measured P concentration and PC (Duru and Thélier-Huché 1997). Values of PNI equal to or greater than 100% indicate that P is not limiting, while values smaller than 100% indicate P deficiency. The PNI is a plant-based diagnostic tool independent from soil types and climate conditions. Along with providing a measure of the level of P nutrition, it also provides information on soil P availability.
Obtaining P limiting conditions in field experiments is often difficult as soils are often rich in available P. For spring wheat and maize (Ziadi et al. 2007, 2008a), the models of PC were first established from experiments with only one non-limiting P fertilization rate. In subsequent studies with contrasted P fertilization rates, there was no response of spring wheat to P fertilization on eight different sites (Bélanger et al. 2015b), while rapeseed responded to P fertilization at only one out of five sites (Bélanger et al. 2015a). Models of PC for wheat, maize, and rapeseed, discussed above, need to be validated under conditions of P deficiencies.
This study focuses on three crops: winter wheat (Triticum hybernum L.), maize (Zea mays L.), and rapeseed (Brassica napus L.) grown in a long-term field experiment with contrasted P mineral fertilization rates conducted in Changins (Switzerland). The objectives were to (1) evaluate the effect of long-term (> 40 years) P fertilization on the grain yield of winter wheat, maize, and rapeseed, (2) validate or establish models of PC based on relationships of shoot P concentration with either shoot biomass DM yield or shoot N concentration under contrasted P conditions including P deficiency, and (3) assess both plant-based and soil-based diagnostic tools for determining the need for P fertilization. This unique context of large variations in soil P contents due to very long-term contrasted P fertilization will contribute to the development of better predictors of P requirements.
Materials and methods
Site description and experimental design
A field experiment was conducted at Agroscope in Changins (VD, Switzerland; 46°23′55.72′′N, 06°14′24.72′′E; altitude 432 m) on a Gleyic Cambisol (FAO classification system) with 525 g clay kg−1 and 163 g sand kg−1 in the top 0.20-m soil layer. A 4-year rotation of rapeseed, winter wheat, maize, and winter wheat was initiated in 1971. Winter wheat was replaced by sugar beet (Beta vulgaris L.) in 1982, and by spring wheat in 1983, 1993, and 2001. The soil was plowed to a depth of 0.20–0.25 m from 1971 to 1985 and only disked to 0.12–0.15 m with a harrow after 1985. Crop residues were left on the field after harvest.
Each year, five fertilization treatments were applied: (1) 0P0K: no P and K applied; (2) 0PK: no P applied and K applied in quantities equivalent to the theoretical crop uptake; (3) P0K: P applied in quantities equivalent to the theoretical crop uptake and no K applied; (4) PK: P and K applied in quantities equivalent to the theoretical crop uptake; (5) PK > exp.: P and K applied in quantities equivalent to double the theoretical nutrient uptake. The theoretical uptake is calculated from averaged local grain yield and nutrient concentration data for most Swiss crops. It is regularly updated and used as reference value in the Swiss official fertilization guidelines (Sinaj et al. 2017). The P and K fertilizers were respectively applied as triple superphosphate [Ca(H2PO4)2] and salt of potash (KCl) prior to plowing or disking for all three crops. The five treatments were set in a randomized complete block design with four replications. Plot size was 15 × 8 m with a 1-m separation between plots. Nitrogen was applied as ammonium nitrate (NH4NO3) at the same rate in all fertilization treatments according to the Swiss fertilization guidelines for each crop species (Sinaj et al. 2017). Herbicides were applied depending on weed infestation, and standard phytosanitary protection was applied according to integrated crop protection principles (Häni et al. 1990). Potassium in 0K treatments was considered non-limiting in this study, given the initially very high soil K content (data not shown).
The current study was undertaken in 2010. Winter wheat (cv. Arina) was sown (180 kg seeds ha−1) on 8 October 2010 and harvested on 12 July 2011, maize (cv. Ricardinio) was sown (94,000 seeds ha−1) on 15 May 2012 and harvested on 1 October 2012, while rapeseed (cv. Visby) was sown (500,000 seeds ha−1) on 30 August 2013 and harvested on 6 July 2014.
Mean annual temperature and precipitation were, respectively, 9.2 °C and 999 mm for the period 1961–1990, and 10.2 °C and 954 mm for the period 1981–2010. The mean annual precipitation was below the 30-year averages (810 mm) in 2011 but above in 2012 (1199 mm) and 2014 (1287 mm). All 3 years were warmer than the 30-year averages, with mean annual temperatures of 11.8, 11.1, and 11.8 °C in 2011, 2012, and 2014, respectively.
Soil sampling and analysis
Soil samples (0–0.20 m and 0.20–0.50 m) were collected for general characteristics analysis in August 2014 after the rapeseed harvest, and in 2011, 2012 and 2014 for available P analysis. At least eight cores with a diameter of 2.5 cm were taken randomly from each plot. Plant residues were removed from the soil and individual core samples were mixed to form one composite soil sample per plot. Soil samples were air-dried and sieved (≤ 2 mm) prior to analysis. Soil total P (Total P) was obtained by digestion of 0.25 g of soil previously treated in 5 ml of hydrofluoric acid (40%) and 1.5 ml of HClO4 (65%) according to the AFNOR standard X31-147 (1996) followed by molybdate colorimetric measurement (Murphy and Riley 1962). Soil available P was evaluated by three methods used in routine analyses. The first method operates at an acidic pH (4.6) in the presence of ammonium acetate and EDTA as a complexing agent (FAL et al. 2004, P-AAE), the second operates at an alkaline pH (8.5) with sodium bicarbonate (Olsen et al. 1954, P-NaHCO3), while the third operates at soil pH and at a very low ionic strength (FAL et al. 2004, P-CO2). The P-AAE and P-CO2 are the two methods used in routine soil available P tests in Switzerland (Sinaj et al. 2009), whereas the P-NaHCO3 method is the most widely used indicator for soil available P worldwide.
Plant sampling and analysis
Shoot biomass was collected weekly on a 1-m2 area in each plot using pruning shears during 7 weeks between tillering and joint stage (CD23–31 to CD45 in BBCH scale, Meier 2001) for winter wheat in 2011, during 6 weeks between leaf development and senescence (CD14–16 to CD99) for maize in 2012, and during 8 weeks between inflorescence emergence and ripening (CD53–55 to CD85) for rapeseed in 2014. At harvest, grain yield was measured in each plot on an area of 31.5 m2 for maize and rapeseed, and 29.5 m2 for winter wheat.
Shoot biomass samples were weighed before and after shredding and oven-drying at 55 °C for 72 h. Thereafter, the samples were ground in a Retsch rotor mill. Residual humidity was evaluated at 103 °C. Dry ashes and organic matter were evaluated by calcination (480 °C). Total N was measured after combustion, using the Dumas method (Masson et al. 2010). Total P was determined by radial ICP-AES (Varian Vista RL Simultaneous or Varian 725ES Simultaneous) after incineration (480 °C for 5 h) and solubilization in hydrofluoric acid (Masson et al. 2010). Crop grain quality was analyzed by Near Infrared Spectroscopy (NIRS) for the determinations of grain protein concentration for winter wheat, maize and rapeseed, and grain oil concentration for rapeseed.
Statistical analysis, calculations, and modeling
Data were checked for normality and variance homoscedasticity. Shoot biomass, shoot P and N concentrations, grain yield, and grain P, protein and oil concentration were subjected to an analysis of variance (ANOVA) with fertilization treatments as a fixed effect and replicates as a random effect. Mean differences between pairs of fertilization treatments were evaluated by a Tukey HSD test. All statistics and model calculations were performed with R software package (R Development Core Team 2011).
Shoot growth was analyzed with a linear parallel curve analysis with grouped data, in which any difference in the response curves of shoot biomass to the number of days from the first day of sampling among fertilization treatments was determined. Using the FIT directive of GENSTAT (VSN International 2011), the response curves were described by the following models:
where SB is the response variable, Days is the explanatory variable, and a and b are the estimated intercept and slope parameters. The procedure initially calculated one equation for the five fertilization treatments, which described the average response to the explanatory variable. In the next step, separate a parameters for SB were estimated for each fertilization treatment to determine the vertical distance between parallel lines (i.e., response curves). The following step estimated separate linear parameters b for the slope (i.e., the interaction between the linear portion of the fertilization treatment and the explanatory variable). At each stage, statistical significance was calculated for the change in the mean square explained by the addition of another parameter (fertilization treatments and fertilization treatments by the explanatory variable interaction) to the model. Slopes of the linear regressions between shoot biomass and the number of days are estimates of shoot growth rates. This approach was used to separate the effect of fertilization rates between early season shoot growth up to the first sampling day and rates of shoot growth later in the season (Bélanger et al. 2015b). Statistical significance was assessed at P ≤ 0.05.
Then, the relationship between shoot P concentration and shoot biomass was described by a power function of allometric type:
where Psc is the shoot P concentration (g kg−1 DM), SB is the shoot biomass (t DM ha−1), c is the estimated scaling factor, and d is the estimated power parameter. The relationship between shoot P and N concentrations was described by a linear regression:
where Nsc is the shoot N concentration, e is the estimated slope and f is the estimated intercept. The P dilution in shoot biomass and the P linear relationship with N were calculated for all five treatments in all three crops. Then, the treatment with the lowest P input within the treatments that resulted in maximum grain yield (maize and rapeseed) or maximum shoot growth (winter wheat) was considered closest to critical P concentration and was chosen as reference. Those reference treatments corresponded to PK for winter wheat and rapeseed, and 0PK for maize.
An allometric function was also fitted on N concentration data and compared to NC established in a previous study to evaluate if N nutrition could be considered optimal. Sampling dates when shoot biomass was below 1 t DM ha−1 were excluded because, at these early stages, the relationship between P and N concentrations is not linear (Lemaire and Gastal 1997). Consequently, five sampling dates for winter wheat and maize, and seven sampling dates for rapeseed were used.
To determine which of the two relationships (PC–SB or PC–Nsc) should be used to calculate the PNI, the linear regression between measured and simulated values was estimated for all experimental plots, similar to the approach used by Pineiro et al. (2008). The coefficient of determination (R2) and the root mean square deviation (RMSD) were used to determine the relationship with the best predicting power for calculating the PNI. The PNI for winter wheat and rapeseed was calculated as follows (Lemaire and Salette 1984):
where Pmeasured is the measured shoot P concentration (g kg−1 DM) and PC is the critical P concentration (g kg−1 DM).
Finally, crop relative grain yields were calculated by dividing the grain yield from a given fertilization treatment by the grain yield from the treatment PK and expressed as percent. Maximal grain yield was considered to be achieved in the PK treatment for all three crops. The relationship between relative grain yield (RGY) and P-NaHCO3, P-AAE and P-CO2 for winter wheat, maize and rapeseed was described by the following hyperbolic function that takes in account yield reduction at very high fertilization rate:
where g, h and i are estimated parameters. The intersection between 95% of calculated maximal grain yield and the function was used to determine critical threshold values for P-NaHCO3, P-AAE and P-CO2.
The relationship between RGY and PNI was described by the following quadratic function:
where j, k and l are estimated parameters. The intersection between 95% of maximal grain yield and the function was used to determine a critical threshold value for PNI.
Results and discussion
Soil phosphorus and other soil characteristics after 44 years of fertilization treatments
Phosphorus fertilization significantly affected soil total P concentration and the three indicators of soil available P in the topsoil (0–0.20 m) after 44 years of cultivation and fertilization treatments (Table 1). Soil total P concentration ranged from 0.72 to 1.14 g P kg−1, while soil available P concentration ranged from 0.62 to 7.54 mg P kg−1 for the P-CO2 extraction, from 7.1 to 72.5 mg P kg−1 for the P-AAE extraction, and from 9.7 to 60.7 mg P kg−1 for the P-NaHCO3 extraction. Soil total P and soil available P, evaluated by the three extraction methods, followed similar response patterns to the fertilization treatments (Table 1). Soil pH and CEC as well as soil organic C and total N concentrations were not significantly affected by the fertilization treatments (Table 1). For the subsoil layer (0.20–0.50 m), P fertilization did not significantly affect concentrations of total P (mean value of 0.40 g kg−1), P-CO2 (0.09 mg kg−1), organic C (11.4 g kg−1), and pH (7.10). However, the PK > exp treatment with annual applications of 52.4 kg P ha−1 significantly differed from all other fertilization treatments for soil available P extracted by NaHCO3 (7.52 vs. 3.54 mg kg−1 across all other treatments) and AAE (2.96 vs. 1.41 mg kg−1), indicating a downward movement to the solid phase of the subsoil. The cumulative P budget over the 44 years of the experiment was positive with the treatments having received P fertilization equivalent to theoretical crop P uptake (PK and P0K) (Table 1). The P input was therefore greater than P exported in the grain crops. Potassium fertilization did not affect any of the soil characteristics, except for a slight increase in soil available K extracted by AAE and CO2 (data not shown), and total K (Table 1) in the 0–0.20 m soil layer with the PK > exp treatment.
Crop growth, and grain yield and quality
The shoot biomass of winter wheat in 2011 was significantly affected by the P fertilization treatments on all seven sampling weeks (Table 2) with generally greater shoot biomass when P was applied (P0K, PK, and PK > exp) than with no applied P (0P0K and 0PK). The fertilization treatments significantly affected the shoot biomass of maize in 2012 only in the first two sampling weeks (stage CD14–16; Meier 2001) and the shoot biomass of rapeseed in 2014 only in the fifth and sixth sampling weeks (CD53–63). Shoot growth was analyzed with a linear parallel curve analysis that indicated different crop responses to the P fertilization treatments. For rapeseed, the additional mean square contributed by Treatments was significant, while that contributed by Days × Treatments was not significant (Table 3). Hence, the regression lines of shoot biomass as a function of time for the different P rates were parallel with similar slopes and growth rates. Most of the effect of the P fertilization treatments on the shoot growth of rapeseed therefore occurred before the first sampling week (Table 3). Similar results were reported from a study with rapeseed conducted at two sites in Canada (Bélanger et al. 2015a). The results presented here and those of Bélanger et al. (2015a) confirm that an adequate P supply during early rapeseed growth is important (Grant et al. 2001, 2009). For winter wheat, the additional mean square contributed by both Treatments and Days × Treatments was significant. Hence, both shoot growth before the first sampling week and shoot growth during the seven weeks of sampling were affected by the P fertilization treatments. Other studies also showed that P nutrition is important throughout the growing season of winter wheat (Miller et al. 1994). For maize, the additional mean square contributed by both Treatments and Days × Treatments was not significant.
Grain yields with the PK treatment were similar to or slightly greater than the average yield since 1995 for winter wheat (5.54 vs. 4.68 t ha−1) and maize (8.37 vs. 8.03 t ha−1). The rapeseed grain yield in 2014, however, was lower than the average yield since 1995 (2.60 vs. 3.11 t ha−1), perhaps due to late planting or seasonally high precipitation in the winter and spring of 2014 (288 mm between January and March) that prevented an herbicide application, causing weed competition. It might also have caused nutrient leaching, creating limiting N conditions.
The fertilization treatments significantly affected the grain yield of winter wheat and rapeseed, but they did not affect the grain yield of maize (Table 4). For winter wheat, the 0P0K treatment resulted in significantly lower grain yields than the treatments with the recommended P rate (PK and P0K). This result contrasts with several short-term studies reporting no wheat grain yield response to P fertilization (McKenzie et al. 2003, 2008; Valkama et al. 2009, 2011; Grant et al. 2009). More recently, in an eight site-year study conducted with contrasting P rates, Bélanger et al. (2015b) reported no spring wheat grain yield response to increasing P fertilization. The rapeseed grain yield for the treatments with no P (0P0K and 0PK) was less than that with applied P (P0K and PK). Positive grain yield responses to P fertilization were reported by Brennan and Bolland (2007) in 21 out of 22 experimental sites and by Bélanger et al. (2015b) in two out of five experimental sites, thus demonstrating the sensitivity of rapeseed grain yield to P deficiency. The absence of a grain yield response of maize after 44 years of P fertilization is probably due to its low crop P requirements or its deep rooting and P uptake from deeper layers (Boniface and Trocmé 1988; Gill et al. 2005). Numerous long-term experiments have reported no or little response of maize to P fertilization (Gallet et al. 2003; Magyar et al. 2006; Messiga et al. 2010).
Although not statistically significant, the treatment with the highest P rate (PK > exp) resulted in slightly but systematically lower grain yield compared to the treatments with the recommended P rate (PK, P0K) for all three crops (Table 4). This trend may be partly explained by antagonism or competition among nutrients. High soil P has been reported to decrease zinc (Zn) uptake in several crops including maize (Marschner 1995; Izsaki 2014). A Zn deficiency was observed in maize in 2012 at the beginning of the leaf development stage (CD11). Even though Zn was applied [foliar application (700 g ha−1)] in the form of zinc oxide (ZnO) at stage CD16 (6 leaves) to limit yield variation due to Zn deficiency, plants at harvest still had lower Zn concentrations for the PK > exp treatment (data not shown) than for the treatments with the recommended P rate.
The grain P concentration of all three crops was significantly positively affected by the fertilization treatments (Table 4). Plants from over-fertilized treatments have achieved a “luxury consumption”, which is defined by the fact that not all P taken up by the plants was used for biomass production. The grain protein concentration of winter wheat and rapeseed was significantly negatively affected by fertilization treatments, while that of maize was not affected. The grain protein concentration tended to be lower with increasing applied P most likely because of N dilution in the increasing grain biomass (Russell et al. 1958; Daccord et al. 2002). Although the fertilization treatments affected the grain protein concentration of winter wheat and rapeseed (Table 4), the total protein produced per hectare (data not shown) was not affected. These results are similar to those of Holford et al. (1992) who reported a decrease of protein concentration with repeated P applications. The oil concentration of rapeseed ranged from 522 to 528 g kg−1 DM and was not significantly affected by P fertilization, which is in agreement with the results of Brennan and Bolland (2007).
Critical P concentration defined as a function of shoot biomass
Shoot P concentration decreased with increasing shoot biomass for the three crops and fertilization treatments (Fig. 1). Models of shoot PC were determined using the data of shoot biomass and P concentration from treatments resulting in maximum shoot biomass growth with the lowest P fertilization rate: PK in wheat, 0PK in maize, and PK in rapeseed. The relationship between PC and shoot biomass (SB) for the three crops were closely described by allometric functions:
Shoot P concentration as a function of shoot biomass (SB) for five fertilization treatments along with the estimated model of PC for a winter wheat (PCww = 4.44 × SB−0.41, R2 = 0.95), b maize (PCmaize = 3.49 × SB−0.19, R2 = 0.99), and c rapeseed (PCrapeseed = 5.18 × SB−0.39, R2 = 0.89) under non-limiting P conditions. For wheat, critical P concentrations expressed as a function of shoot biomass are presented for four locations from Bélanger et al. (2015a, b). 0P0K: no P and K applied; 0PK: no P applied, K applied in quantities equivalent to crop uptake; P0K: P applied in quantities equivalent to crop uptake and no K applied); PK: P and K applied in quantities equivalent to crop uptake); PK > exp: P and K applied in quantities equivalent to crop uptake with additional fertilization of 26.2 kg P ha−1 and 166 kg K ha−1
The model obtained in this study for winter wheat predicted PC values close to the lowest values predicted by Bélanger et al. (2015b) (Fig. 1). Luxury consumption at some of the locations studied by Bélanger et al. (2015b) might be responsible for the variations observed in their study. The relationship between PC and shoot biomass has never been tested in maize and rapeseed. Current results therefore provide a first estimation of their PC as a function of shoot biomass. Because of the lack of a grain yield response to P fertilization, PC of maize may be over-estimated.
For shoot biomass less than 1 t DM ha−1, a constant PC for winter wheat (3.9 g kg−1 DM) and maize (2.6 g kg−1 DM) was assumed and calculated as the mean P concentration from the reference treatments, PK for winter wheat and 0PK for maize, as did Lemaire and Gastal (1997). To have a more precise biomass threshold value where the P dilution phase starts, we calculated the intersection between these constant initial values of PC and the modeled dilution curve of PC, as suggested by Justes et al. (1994) for N dilution in winter wheat. This analysis indicates that P dilution starts approximately at 1.36 t DM ha−1 for winter wheat and 1.34 t DM ha−1 for maize.
Although a strong relationship between PC and shoot biomass was found, its use for assessing the crop P status might be hampered by other factors. For example, Bélanger et al. (2015b) concluded that the allometric relationship between PC and shoot biomass in wheat differed among locations and, consequently, the wide use of the PC model expressed as a function of shoot biomass is limited. Furthermore, previous research on perennial grasses (Bélanger and Ziadi 2008) has shown that the relationship between PC and shoot biomass varied with the level of N nutrition. More research is needed to determine this variation of the PC–SB relationship with a range of N nutrition in maize, winter wheat, and rapeseed.
Critical P concentration defined as a function of shoot N concentration
Because the relationship between critical P concentration and N concentration is affected by the level of N nutrition (Ziadi et al. 2007, 2008a), we first evaluated the level of N nutrition of the reference treatments (PK for winter wheat and rapeseed, and 0PK for maize) by fitting allometric functions to our data, and comparing them with curves of shoot critical N concentration (NC) previously published on wheat by Ziadi et al. (2010; NC = 38.5 × SB−0.57), Greenwood et al. (1987; NC = 38.6 × SB−0.44) and Justes et al. (1994; NC = 53.5 × SB−0.44), on maize by Plénet and Lemaire (2000; NC = 34.0 × SB−0.37) and Herrman and Taube (2004; NC = 34.0 × SB−0.39), and on rapeseed by Colnenne et al. (1998; NC = 44.8 × SB−0.25) (Fig. 2). Our allometric functions were as follows:
where Nww and Nrapeseed are respectively the N concentrations of winter wheat and rapeseed for the PK treatment, and Nmaize is the N concentration of maize for the 0PK treatment. Values of Nww in this experiment were comparable to NC predicted by Ziadi et al. (2010), while values of Nmaize were only slightly lower than NC predicted by Plénet and Lemaire (2000) and Herrman and Taube (2004). The N nutrition of winter wheat and maize was thus considered optimum or near optimum, while the N nutrition of rapeseed was below the optimal N nutrition proposed by Colnenne et al. (1998).
Shoot N concentration as a function of shoot biomass (SB) for a winter wheat (Nww = 44.0 × SB−0.59, R2 = 0.93), b maize (Nmaize = 30.6 × SB−0.46, R2 = 0.84) and c rapeseed (Nrapeseed = 57.0 × SB−0.75, R2 = 0.89) for five fertilization treatments, as compared to critical N concentrations determined by Greenwood et al. (1990) and Ziadi et al. (2010) for winter wheat, Herrman and Taube (2004) and Plénet and Lemaire (2000) for maize, and Colnenne et al. (1998) for rapeseed. 0P0K: no P and K applied; 0PK: no P applied, K applied in quantities equivalent to crop uptake; P0K: P applied in quantities equivalent to crop uptake and no K applied); PK: P and K applied in quantities equivalent to crop uptake); PK > exp: P and K applied in quantities equivalent to crop uptake with additional fertilization of 26.2 kg P ha−1 and 166 kg K ha−1
Shoot P concentration increased with increasing shoot N concentration for all three crops and fertilization treatments (Fig. 3). As for the relationship between PC and shoot biomass, models of PC as a function of shoot N concentration were determined with data of shoot P and N concentrations with maximum shoot growth and lowest P fertilizer application rate: PK in wheat, 0PK in maize, and PK in rapeseed. The PC models were:
Shoot P concentration as a function of shoot N concentration for five fertilization treatments along with the critical P concentrations expressed as a function of shoot N concentration for a winter wheat with a linear (PCww = 0.083 N + 0.88, R2 = 0.85) and a quadratic model (PCww = 0.291 N − 1.557 − 0.004 N2, R2 = 0.98), b maize (PCmaize = 0.083 N + 0.39, R2 = 0.92) and c rapeseed (PCrapeseed = 0.657 N + 1.67, R2 = 0.85). Previously published models for wheat [Ziadi et al. (2008a), PCw = 0.107 N + 0.94; Bélanger et al. (2015a, b), PCw = 0.221 N − 0.677 − 0.00292 N2)], maize [(Ziadi et al. (2007), PCmaize = 0.094 N + 1.0)], and rapeseed [Bélanger et al. (2015a, b), PCrapeseed = 0.094 N + 1.0)] are also presented. 0P0K: no P and K applied; 0PK: no P applied, K applied in quantities equivalent to crop uptake; P0K: P applied in quantities equivalent to crop uptake and no K applied); PK: P and K applied in quantities equivalent to crop uptake); PK > exp: P and K applied in quantities equivalent to crop uptake with additional fertilization of 26.2 kg P ha−1 and 166 kg K ha−1
Ziadi et al. (2008a) also determined a linear model of PC as a function of shoot N concentration (PC = 0.18 N + 0.94) for spring wheat. Their initial model probably over-estimated PC because of plant luxury consumption, as no limiting P situations were identified in their experiment. Subsequently, Bélanger et al. (2015b) proposed a quadratic relationship between PC and shoot N concentration (PC = 0.221 N − 0.677 − 0.003 N2, R2 = 0.82, P < 0.001) in an experiment with several P rates over several locations. Even though grain yield in the latter study barely responded to P fertilization, results from our study were similar to those reported by Bélanger et al. (2015b).
The present model for maize predicts lower PC values than those predicted by the model (PC = 0.094 N + 1.0) proposed by Ziadi et al. (2007). Luxury P consumption probably also occurred in their study. This implies that the PC model for maize developed in this study should be validated with a dataset of crops responding to P fertilization in order to ensure no bias due to luxury consumption.
The model obtained in this study for rapeseed predicted greater PC values than those predicted by the model (PC = 0.024 N + 1.74) proposed by Bélanger et al. (2015a). Differences between the PC models can be explained by different cultivars used or by the larger than average precipitation observed in our study which may have caused N leaching and reduced crop growth, in addition to increased weed competition for N because of the absence of an herbicide treatment.
Results presented here from three crops grown under conditions of contrasted soil available P achieved after more than 40 years of fertilization treatments confirm the validity of the models used in previous studies to describe the relationships between shoot P concentration and either shoot biomass or shoot N concentrations. Furthermore, the model of PC for wheat based on the PC–shoot N concentration relationship was in agreement with the model developed by Bélanger et al. (2015b). For maize and rapeseed, however, more data are required to validate the respective PC models.
Agronomic use of PC and PNI
Two relationships based on the concomitant decrease of P and N concentrations with increasing shoot biomass were considered in our assessment of critical P concentration models for three important crops. Previous studies have shown that the PC–shoot N concentration model was more universal than the PC–SB model because the same model parameters could be used for several locations (Bélanger et al. 2015b; wheat) or for several levels of N nutrition (Bélanger and Ziadi 2008; perennial grasses). Furthermore, the PC–shoot N concentration model does not require the determination of the shoot biomass, which makes it more practical for use in farmers’ fields. The two models were also analyzed by comparing measured and predicted PC values (Fig. 4). The PC–shoot N concentration model appears, for all three crops, to predict PC values with less dispersion around the 1:1 line (R2 = 0.93) as opposed to the PC–SB model (R2 = 0.90). For all those reasons, the PC–shoot N concentration model was chosen for calculating the PNI for winter wheat and rapeseed. Maize did not respond significantly to P fertilization and, therefore, the proposed model possibly overestimates PC and potentially underestimates PNI.
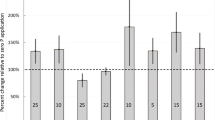
The PNI of winter wheat ranged from 72 to 132%, while that of rapeseed ranged from 60 to 132% (Table 5). The relative grain yield increased with increasing PNI up to about 100% and then decreased, likely because of antagonism with zinc (Fig. 5). According to this relationship, 95% of maximum grain yield would be achieved with PNI of 82% for winter wheat and 81% for rapeseed. Because P fertilization cannot alleviate an early-season P deficiency (Barry and Miller 1989), producers would most likely use the PNI values to adjust fertilization for the following growing season. As suggested by Bélanger et al. (2015a), the PNI values could also be used for an a posteriori diagnosis aimed at detecting limiting factors for crops in experimental trials or production fields.
Relationship between relative grain yield (RGY) and the phosphorus nutrition index (PNI) (mean for all sampling dates) for winter wheat [RGY = − 0.012PNI2 + 2.46PNI − 26.4, Residual Standard Error (RSE) = 7.88, n = 5] and rapeseed [RGY = − 0.015PNI2 + 3.08PNI − 54.1, RSE = 7.37, n = 5]. Relative grain yields of winter wheat and rapeseed were calculated by dividing the grain yield from a given fertilization treatment by the grain yield from the PK treatment. The dashed horizontal line represents a relative grain yield of 95%
Soil analysis
The relationship between relative grain yield and P-NaHCO3, the most widely used method for estimating soil available-P, and P-AAE and P-CO2, the official methods used in Switzerland in routine soil available-P tests, was described by a hyperbolic function for each crop (Fig. 6). This function differs from usual relationships that describe a relatively long plateau phase such as in the Mitscherlich model (Valkama et al. 2011). A deficiency in other nutrients such as Zn (Marschner 1995) due to an antagonism with P from over-fertilization may explain the decline in relative yield with high soil available P values. Minimum threshold values indicating optimal plant nutrition were calculated as the intersection between 95% maximum yield and the hyperbolic curve fitting the relationship between crop yield and soil available P. Threshold values for winter wheat, maize, and rapeseed were respectively 0.33, 0.31 and 0.70 mg P kg−1 soil for P-CO2, 14.7, 11.9 and 15.6 mg P kg−1 soil for P-NaHCO3, and 12.8, 10.0 and 15.2 mg P kg−1 soil for P-AAE. These results are in accordance with that of Messiga et al. (2010) who found a critical value for maize of 8 mg P-NaHCO3 on a slightly alkaline and sandy loamy soil. For winter wheat, Bollons and Barraclough (1999) reported a threshold of 9 mg P-NaHCO3 kg−1 soil on a silty-clay loam soil, whereas Morel et al. (1992), analyzing several French experimental sites, found a critical value that ranged from 6 to 12 mg P-NaHCO3 kg−1 soil depending on the soil type. Johnston et al. (1986) found a critical value of 11 and 18 mg P-NaHCO3 kg−1 soil on a sandy-clay loam for two different cultivars, and of 10, 12 and 8 mg kg−1 on two silty clay loams and one sandy clay loam respectively, for 98% of maximum yield (Johnston et al. 2014). Brennan and Bolland (2007) assessed a critical P-NaHCO3 value for rapeseed of 20 mg kg−1 soil for the 0.01–0.10 m layer of various sandy soils of Australia.
Relationship between relative grain yield (RGY) and a P-NaHCO3 for winter wheat [RGY = 139.6x/(x + 2.81) − 0.66x, residual standard error (RSE) = 7.28, n = 20], maize (RGY = 120.8x/(x + 3.25) − 0.27x, RSE = 11.39, n = 19), and rapeseed [RGY = 144.1x/(x + 5.76) − 0.66x, RSE = 7.19, n = 20], b P-AAE for winter wheat [RGY = 110.2x/(x + 1.72) − 0.40x, RSE = 6.91, n = 20], maize [RGY = 116.7x/(x + 1.77) − 0.66x, RSE = 11.29, n = 19], and rapeseed [RGY = 144.14x/(x + 5.8) − 0.66x, RSE = 9.29, n = 20)], c P-CO2 for winter wheat [RGY = 111.5x/(x + 0.05) − 6.27x, RSE = 7.83, n = 20], maize [RGY = 136.4x/(x + 0.1x) − 29.69x, RSE = 11.09, n = 19], and rapeseed [RGY = 165.8x/(x + 0.38) − 20.04x, RSE = 9.24, n = 20]. Relative grain yields of winter wheat and rapeseed were calculated by dividing the grain yield from a given fertilization treatment by the grain yield from the PK treatment
To evaluate if the specific conditions of 2011–2014 data can be considered representative for this field, we analyzed the long-term relationship between yield and soil available-P, this time with the data collected throughout all 44 years of contrasted P fertilization. Calculated threshold for winter wheat, maize and rapeseed respectively are 0.42, 0.34 and 0.45 mg P kg−1 soil for P-CO2, and 22.3, 12.9 and 18.0 mg P kg−1 soil for P-AAE (supplementary figure 1). This confirms the accuracy of the critical soil P thresholds for P-CO2 and P-AAE calculated based on single-year data, despite the inevitable yearly variability.
Relationships between PNI, as an average of all sampling time points, and the three indicators of soil available P are shown on supplementary figure. The relationship is well described by a power function for all three crops. Winter wheat and maize start to reach a plateau at high soil available P values, showing that luxury consumption is also decreasing with each added unit of P-fertilizer.
Under our experimental conditions, the Swiss fertilizer recommendations (Sinaj et al. 2017) were found not to be adapted to crop specific requirements. Instead, the guidelines suggest no fertilization application for winter wheat and maize from 80 mg P-AAE kg−1 soil only, while the fertilization response thresholds in this study for wheat, maize, and rapeseed were respectively 22, 13 and 18 mg P-AAE kg−1 soil. Same conclusion with P-CO2 for which official guidelines recommend no fertilization from 1.55 mg P-CO2 kg−1 soil, whereas our data indicate that 0.40, 0.30 and 0.50 mg P-CO2 kg−1 soil is enough for winter wheat, maize and rapeseed, respectively. These results suggest that similar adjustments might be needed for other crops and soil types.
Conclusions
Long-term P fertilization applied over a period of 44 years affected soil available P, and the shoot growth and grain yield of winter wheat and rapeseed measured in the last 4 years but had not effect on maize shoot growth and grain yield. The relationships between PC and shoot biomass or N concentration were very good (R2 > 0.85) for all three crops. The PC–shoot N concentration model for winter wheat from this study confirmed results from previous studies and can be used for calculating PNI. For maize and rapeseed, however, further research is required to ensure situations with no P luxury consumption and N deficiency. Moreover, there is unavoidable variability from year to year and between local conditions and soil types, which makes further studies including multiple sites and years necessary to confirm and ameliorate the precision of PC values.
Based on the relationship between grain yield and three indicators of soil availability, threshold values for 95% of the maximum yield for winter wheat, maize, and rapeseed were less than those currently used in the official fertilization guidelines in Switzerland. Our results indicate that the refinement of fertilization requirements with more sensitive diagnostic tools that take into account the long-term effects of continuous fertilization could result in substantial reductions in fertilizer application rates.
References
AFNOR (1996) NF X31-147: Qualité des sols: sols sédiments, mise en solution totale par attaque acide. AFNOR, La Plaine Saint-Denis Cedex
Barry DAJ, Miller MH (1989) Phosphorus nutritional requirement of maize seedlings for maximum yield. Agron J 81:95–99
Bélanger G, Ziadi N (2008) Phosphorus and nitrogen relationships during spring growth of an aging timothy sward. Agron J 100:1757–1762
Bélanger G, Ziadi N, Pageau D, Grant C, Lafond J, Nyiraneza J (2015a) Shoot growth, phosphorus–nitrogen relationships, and yield of canola in response to mineral phosphorus fertilization. Agron J 107:1458–1464
Bélanger G, Ziadi N, Pageau D, Grant C, Hognasbacka M, Virkajarvi P, Hu Z, Lu J, Lafond J, Nyiraneza J (2015b) A model of critical phosphorus concentration in the shoot biomass of wheat. Agron J 107:963–970
Bélanger G, Ziadi N, Lajeunesse J, Jouany C, Virkajarvi P, Sinaj S, Nyiraneza J (2017) Shoot growth and phosphorus–nitrogen relationship of grassland swards in response to mineral phosphorus fertilization. Field Crops Res 204:31–41. https://doi.org/10.1016/j.fcr.2016.12.006
Bollons HM, Barraclough PB (1999) Assessing the phosphorus status of winter wheat crops: inorganic orthophosphate in whole shoots. J Agric Sci 133:285–295. https://doi.org/10.1017/S0021859699007066
Boniface R, Trocmé S (1988) Enseignements fournis par des essais de longue durée sur la fumure phosphate et potassique. Essais sur la fumure phosphatée. In: Gachon L (ed) Phosphore et potassium dans les relations sol-plante: conséquences sur la fertilisation. Inra Editions, Paris, pp 279–401
Brennan RF, Bolland MDA (2007) Effect of fertiliser phosphorus and nitrogen on the concentrations of oil and protein in grain and the grain yield of canola (Brassica napus L.) grown in south-western Australia. Aust J Exp Agric 47:984–991. https://doi.org/10.1071/EA06115
Carpenter SR (2005) Eutrophication of aquatic ecosystems: bistability and soil phosphorus. Proc Natl Acad Sci 102:10002–10005. https://doi.org/10.1073/pnas.0503959102
Colnenne C, Meynard JM, Reau R, Justes E, Merrien A (1998) Determination of a critical dilution curve for winter oilseed rape. Ann Bot (Lond) 81:311–317
Daccord R, Arrigo Y, Jeangros B, Scehovic J, Schubiger FX, Lehmann J (2002) Valeur nutritive des plantes des prairies/Valeurs azotées et énergétiques. Rev Suisse Agric 34:73–78
Demaria P, Flisch R, Frossard E, Sinaj S (2005) Exchangeability of phosphate extracted by four chemical methods. J Plant Nutr Soil Sci 168:89–93. https://doi.org/10.1002/jpln.200421463
Duru M, Thélier-Huché L (1997) N and P-K status of herbage: use for diagnosis on grasslands, diagnostic procedures for crop N management. Les Colloques de l’INRA 82:125–138
FAL, RAC, FAW (2004) Méthodes de référence des stations fédérales de recherches agronomiques. Agroscope, vol 2, Zürich-Reckenholz, Switzerland
Gallet A, Flisch R, Ryser JP, Frossard E, Sinaj S (2003) Effect of phosphate fertilization on crop yield and soil phosphorus status. J Plant Nutr Soil Sci 166:568–578
Gilbert N (2009) The disappearing nutrient. Nature 461:716–718
Gill AS, Sadana US, Samal D (2005) Phosphorus influx and root-shoot relations as indicators of phosphorus efficiency of different crops. Commun Soil Sci Plant Anal 36:2315–2327
Grant CA, Flaten DN, Tomasiewicz DJ, Sheppard SC (2001) The importance of early season phosphorus nutrition. Can J Plant Sci 81:211–224
Grant CA Montreal, Irvine MA, Mohr RB, McLaren RM, Khakbzan DL (2009) Crop response to current and previous season applications of phosphorus as affected by crop sequence and tillage. Can J Plant Sci 89:49–66
Greenwood DJ, Verstraeten LMJ, Draycott A (1987) Response of winter wheat to N-fertiliser: quantitative relations for components of growth. Fertil Res 12:119–137
Greenwood DJ, Lemaire G, Gosse G, Cruz P, Draycott A, Neeteson JJ (1990) Decline in percentage N of C3 and C4 crops with increasing plant mass. Ann Bot 66:425–436
Häni F, Popow G, Reinhard H, Schwarz A, Tanner K, Vorlet M (1990) Protection des plantes en production intégrée. LMZ Centrale des moyens d’enseignement agricole, Zollikofen
Haygarth PM, Jarvis SC (1999) Transfer of phosphorus from agricultural soils. Adv Agron 66(66):195–249
Herrmann A, Taube F (2004) The range of the critical N dilution curve for maize (Zea mays L.) can be extended until silage maturity. Agron J 96:1131–1138
Holford ICR, Doyle AD, Leckie CC (1992) Nitrogen response characteristics of wheat protein in relation to yield responses and their interactions with phosphorus. Aust J Agric Res 43:969–986
Izsaki Z (2014) Effects of phosphorus supplies on the nutritional status of maize (Zea mays L.). Commun Soil Sci Plant Anal 45:516–529
Johnston AE, Lane PW, Mattingly GEG, Poulton PR, Hewitt MV (1986) Effect of soil and fertilizer P on yields of potatoes, sugar beet, barley and winter wheat on a sandy clay loam soil at Saxmundham, Suffolk. J Agric Sci 106:155–167
Johnston AE, Poulton PR, Fixen PE, Curtin D (2014) Phosphorus: its efficient use in agriculture. Adv Agron 123:177–229
Justes E, Mary B, Meynard J-M, Machet J-M, Thelier-Huche L (1994) Determination of a critical nitrogen dilution curve for winter wheat crops. Ann Bot (Lond) 74:397–407
Lemaire G, Gastal F (1997) N uptake and distribution in plant canopies. In: Lemaire G (ed) Diagnosis of the nitrogen status in crops. Springer, Berlin, pp 3–43
Lemaire G, Salette J (1984) Relation entre dynamique de croissance et dynamique de prélèvement d’azote pour un peuplement de graminés fourragères. I—Etude de l’effet du milieu. Agronomie 4:423–430
Magyar M, Csatho P, Debreczeni K, Sardi K (2006) Evaluation of agronomic and environmental soil P test methods in a network of Hungarian long-term field trials. Commun Soil Sci Plant Anal 37:2423–2446
Marschner M (1995) Mineral nutrition of higher plant, 2nd edn. Academic Press, London
Masson P, Dalix T, Bussiere S (2010) Determination of major and trace elements in plant samples by inductively coupled plasma-mass spectrometry. Commun Soil Sci Plant Anal 41:231–243
McKenzie RH, Bremer E, Kryzanowski L, Middleton AB, Solberg ED, Heaney D (2003) Yield benefit of phosphorus fertilizer for wheat, barley and canola in Alberta. Can J Plant Sci 83:431–441
McKenzie RH, Middleton AB, Dunn R, Sadasivaiah RS, Beres B, Bremer E (2008) Response of irrigated soft white spring wheat to seeding date, seeding rate and fertilization. Can J Plant Sci 88:291–298
Meier U (2001) Stades phénologiques des mono- et dicotylédones cultivées—BBCH Monographie. Centre fédéral de recherches biologiques pour l’agriculture et les forêts, Braunschweig, Deutschland
Messiga AJ, Ziadi N, Plénet D, Parent L-É, Morel C (2010) Long-term changes in soil phosphorus status related to P budgets under maize monoculture and mineral P fertilization. Soil Use Manag 26(3):354–364
Messiga AJ, Ziadi N, Jouany C, Virkajärvi P, Soumela R, Sinaj S, Bélanger G, Stroia C, Morel C (2015) Soil test phosphorus and cumulative budgets in fertilized grassland. Ambio 44:252–262
Miller M, McGonigle T, Addy H (1994) An economic approach to evaluate the role of mycorrhizas in managed ecosystems. Plant Soil 159:27–35
Morel C, Plenchette C, Fardeau JC (1992) La fertilisation phosphatée raisonnée de la culture du blé. Agronomie 12:565–579
Murphy J, Riley JP (1962) A modified single solution method for determination of phosphate in natural waters. Anal Chim Acta 26:31–36
Olsen SR, Cole CV, Watanabe FB, Dean LA (1954) Estimation of soil available phosphorus in soils by extraction with sodium bicarbonate. US Department of Agriculture, Circular, Washington, p 939
Pineiro G, Perelman S, Guerschman JP, Paruelo JM (2008) How to evaluate models: observed vs predicted or predicted vs observed? Ecol Model 216(3–4):316–322
Plénet D, Lemaire G (2000) Relationships between dynamics of nitrogen uptake and dry matter accumulation in maize crops. Determination of critical N concentration. Plant Soil 216:65–82
R Development Core Team (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. Accessed 28 Oct 2016
Russell GC, Smith AD, Pittman UJ (1958) The effect of nitrogen and phosphorus fertilizers on the yield and protein content of spring wheat grown on stubble fields in southern Alberta. Can J Plant Sci 38:139–144
Salette J, Lemaire G (1981) Sur la variation de la teneur en azote de graminées fourragères pendant leur croissance: formulation d’une loi de dilution. C R Acad Sci Paris 292:875–878
Sinaj S, Richner W, Flisch R, Charles R (2009) Données de base pour la fumure des grandes cultures et des herbages (DBF-GCH). Rev Suisse Agric 41:98
Sinaj S, Charles R, Baux A, Dupuis B, Hiltbrunner J, Levy L, Peller D, Blanchet G, Jeangros B (2017) Fertilisation des grandes cultures. In: Sinaj S, Richner W (eds) Principes de la fertilisation des cultures agricoles en Suisse, vol 8(6). Posieux, Recherche Agronomique Suisse, pp 10/1–10/16 (publication spéciale)
Valkama E, Uusitalo R, Ylivainio K, Virkajärvi P, Turtola E (2009) Phosphorus fertilization: a meta-analysis of 80 years of research in Finland. Agr Ecosyst Environ 130:75–85
Valkama E, Uusitalo R, Turtola E (2011) Yield response models to phosphorus application: a research synthesis of Finnish field trials to optimize fertilizer P use of cereals. Nutr Cycl Agroecosyst 91:1–15
VSN International (2011) GenStat for windows. 14th ed. VSN Int, Hemel Hempstead, UK. http://www.vsni.co.uk/software/genstat. Accessed on 9 July 2013
Ziadi N, Belanger G, Cambouris AN, Tremblay N, Nolin MC, Claessens A (2007) Relationship between P and N concentrations in corn. Agron J 99:833–841. https://doi.org/10.2134/agronj2006.0199
Ziadi N, Belanger G, Cambouris AN, Tremblay N, Nolin MC, Claessens A (2008a) Relationship between phosphorus and nitrogen concentrations in spring wheat. Agron J 100:80–86. https://doi.org/10.2134/agrojnl2007.0119
Ziadi N, Brassard M, Bélanger G, Cambouris AN, Tremblay N, Nolin MC, Claessens A, Parent L-E (2008b) Critical nitrogen curve and nitrogen nutrition index for corn in eastern Canada. Agron J 100:271–276. https://doi.org/10.2134/agrojnl2007.0059
Ziadi N, Belanger G, Claessens A, Lefebvre L, Cambouris AN, Tremblay N, Nolin MC, Parent L-E (2010) Determination of a critical nitrogen dilution curve for spring wheat. Agron J 102:241–250
Ziadi N, Angers DA, Gagnon B, Lalande R, Morel C, Rochette P, Chantigny MH (2014) Long-term tillage and synthetic fertilization affect soil functioning and crop yields in a corn-soybean rotation in eastern Canada. Can J Soil Sci 94(3):365–376
Acknowledgements
The authors gratefully acknowledge the financial support by Agroscope. Thanks go to Saïd Elfouki and Vincent Bovet for their technical support during sampling operations and field measurements.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Cadot, S., Bélanger, G., Ziadi, N. et al. Critical plant and soil phosphorus for wheat, maize, and rapeseed after 44 years of P fertilization. Nutr Cycl Agroecosyst 112, 417–433 (2018). https://doi.org/10.1007/s10705-018-9956-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-018-9956-0