Abstract
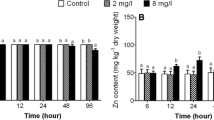
The study was carried out to evaluate the effects of low-dose zinc (Zn) pre-exposure on survival rate, new Zn accumulation, and mitochondrial bioenergetics in the liver and spleen of large yellow croaker exposed to high-dose Zn. To the end, fish were pre-exposed to 0 and 2 mg L−1 Zn for 48 h and post-exposed to 0 and 12 mg L−1 Zn for 48 h. Twelve milligrams Zn per liter exposure alone reduced survival rate, but the effect did not appear in the 2 mg L−1 Zn pre-exposure groups. Two milligrams per liter Zn pre-exposure also ameliorated 12 mg Zn L−1 induced new Zn accumulation, reactive oxygen species (ROS) levels, and mitochondrial swelling in the liver. However, these effects did not appear in the spleen. In the liver, 2 mg L−1 Zn pre-exposure apparently relieved 12 mg L−1 Zn induced down-regulation of activities of ATP synthase (F-ATPase), succinate dehydrogenase (SDH), and malate dehydrogenase (MDH). The mRNA levels of these genes remained relatively stable in fish exposed to 12 mg L−1 Zn alone, but increased in fish exposed to 12 mg L−1 Zn with 2 mg L−1 Zn pre-treatment. In the spleen, 2 mg Zn L−1 pre-exposure did not mitigate the down-regulation of mRNA levels of genes and activities of relative enzymes induced by 12 mg L−1 Zn. In conclusion, our study demonstrated low-dose zinc pre-exposure ameliorated high-dose zinc induced mitochondrial dysfunction in the liver but not in the spleen of large yellow croaker, indicating an organ-specific effect.


Similar content being viewed by others
References
Adeyemi JA, Klerks PL (2013) Occurrence of copper acclimation in the least killifish Heterandria formosa, and associated biochemical and physiological mechanisms. Aquat Toxicol 130:51–57
Ballantyne JS (2004) Mitochondria: aerobic and anaerobic design—lessons from molluscs and fishes. Comp Biochem Physiol B: Biochem Mol Biol 139:461–467
Bourdineaud J-P, Rossignol R, Brethes D (2013) Zebrafish: a model animal for analyzing the impact of environmental pollutants on muscle and brain mitochondrial bioenergetics. Int J Biochem Cell Biol 45:16–22
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cannino G, Ferruggia E, Luparello C, Rinaldi AM (2009) Cadmium and mitochondria. Mitochondrion 9:377–384
Craig PM, Wood CM, McClelland GB (2007) Oxidative stress response and gene expression with acute copper exposure in zebrafish (Danio rerio). Am. J. Physiol. Regul. Integr. Comp. Physiol. 293:R1882–R1892
Dolci G, Vey L, Schuster A, Roversi K, Roversi K, Dias V, Pase C, Barcelos R, Antoniazzi C, Golombieski J (2014) Hypoxia acclimation protects against oxidative damage and changes in prolactin and somatolactin expression in silver catfish (Rhamdia quelen) exposed to manganese. Aquat Toxicol 157:175–185
Drotar A, Phelps P, Fall R (1985) Evidence for glutathione peroxidase activities in cultured plant cells. Plant Sci 42:35–40
Grosell M, McGeer J, Wood CM (2001) Plasma copper clearance and biliary copper excretion are stimulated in copper-acclimated trout. Am J Physiol Regul Integr Comp Physiol 280:R796–R806
Huang Q, Dong S, Fang C, Wu X, Ye T, Lin Y (2012) Deep sequencing-based transcriptome profiling analysis of Oryzias melastigma exposed to PFOS. Aquat Toxicol 120:54–58
Huang GY, Ying GG, Liang YQ, Liu SS, Liu YS (2014) Expression patterns of metallothionein, cytochrome P450 1A and vitellogenin genes in western mosquitofish (Gambusia affinis) in response to heavy metals. Ecotox. Environ. Safe. 105:97–102
James R, Pattu VJ, Devakiamma G, Sampath K (1991) Impact of sublethal levels of mercury on glycogen and selected respiratory enzymes in Heteropneustes fossilis and role of water hyacinth in reduction of Hg toxicity. Ind J Fish 38:249–252
James R, Sampath K, Ponmani KP (1992) Effect of metal mixtures on activity of two respiratory enzymes and their recovery in Oreochromis mossambicus (Peters). Ind J Exp Biol 30:496–499
Jiang Q, Zhang W, Tan H, Pan D, Yang Y, Ren Q, Yang J (2014) Analysis of gene expression changes, caused by exposure to nitrite, in metabolic and antioxidant enzymes in the red claw crayfish, Cherax quadricarinatus. Ecotox Environ Safe 104:423–428
Knapen D, Bervoets L, Verheyen E, Blust R (2004) Resistance to water pollution in natural gudgeon (Gobio gobio) populations may be due to genetic adaptation. Aquat Toxicol 67:155–165
Korshunov SS, Skulachev VP, Starkov AA (1997) High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416:15–18
Landry GM, Dunning CL, Conrad T, Hitt MJ, McMartin KE (2013) Diglycolic acid inhibits succinate dehydrogenase activity in human proximal tubule cells leading to mitochondrial dysfunction and cell death. Toxicol Lett 221:176–184
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2', 7'-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5:227–231
Liu P, Yu Y, Liu C (1991) Studies on the situation of pollution and countermeasures of control of the oceanic environment in Zhoushan fishing ground—the largest fishing ground in China. Mar Poll Bull 23:281–288
Malecot M, Mezhoud K, Marie A, Praseuth D, Puiseux-Dao S, Edery M (2009) Proteomic study of the effects of microcystin-LR on organelle and membrane proteins in medaka fish liver. Aquat Toxicol 94:153–161
Martinez-Reyes I, Cuezva JM (2014) The H+-ATP synthase: a gate to ROS-mediated cell death or cell survival. Biochim Biophys Acta 1837:1099–1112
McGeer JC, Nadella S, Alsop DH, Hollis L, Taylor LN, McDonald DG, Wood CM (2007) Influence of acclimation and cross-acclimation of metals on acute Cd toxicity and Cd uptake and distribution in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 84:190–197
Morin C, Zini R, Simon N, Tillement J (2002) Dehydroepiandrosterone and α-estradiol limit the functional alterations of rat brain mitochondria submitted to different experimental stresses. Neuroscience 115:415–424
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13
Nam YK, Cho YS, Choi BN, Kim KH, Kim SK, Kim DS (2005) Alteration of antioxidant enzymes at the mRNA level during short-term starvation of rockbream Oplegnathus fasciatus. Fish Sci 71:1385–1387
Niu C, Rummer J, Brauner C, Schulte P (2008) Heat shock protein (Hsp70) induced by a mild heat shock slightly moderates plasma osmolarity increases upon salinity transfer in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol C: Pharmacol Toxicol 148:437–444
Onukwufor JO, Kibenge F, Stevens D, Kamunde C (2015) Modulation of cadmium-induced mitochondrial dysfunction and volume changes by temperature in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 158:75–87
Padmini E, Geetha BV, Rani MU (2009) Pollution induced nitrative stress and heat shock protein 70 overexpression in fish liver mitochondria. Sci Total Environ 407:1307–1317
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29:e45–e45
Philip G, Reddy P, Sridevi G (1995) Cypermethrin-induced in vivo alterations in the carbohydrate metabolism of freshwater fish, Labeo rohita. Ecotox. Environm. Safe. 31:173–178
Pujolar J, Milan M, Marino I, Capoccioni F, Ciccotti E, Belpaire C, Covaci A, Malarvannan G, Patarnello T, Bargelloni L (2013) Detecting genome-wide gene transcription profiles associated with high pollution burden in the critically endangered European eel. Aquat Toxicol 132:157–164
Sampaio FG, de Lima Boijink C, Oba ET, dos Santos LRB, Kalinin AL, Rantin FT (2008) Antioxidant defenses and biochemical changes in pacu (Piaractus mesopotamicus) in response to single and combined copper and hypoxia exposure. Comp. Biochem. Physiol. C: Pharmacol. Toxicol. 147:43–51
Sánchez-Aragó M, Formentini L, Cuezva JM (2013) Mitochondria-mediated energy adaption in cancer: the H+-ATP synthase-geared switch of metabolism in human tumors. Antioxid Redox Sign 19:285–298
Sappal R, MacDonald N, Fast M, Stevens D, Kibenge F, Siah A, Kamunde C (2014) Interactions of copper and thermal stress on mitochondrial bioenergetics in rainbow trout, Oncorhynchus mykiss. Aquat Toxicol 157:10–20
Sarkar S, Mukherjee S, Chattopadhyay A, Bhattacharya S (2014) Low dose of arsenic trioxide triggers oxidative stress in zebrafish brain: expression of antioxidant genes. Ecotox Environm Safe 107:1–8
Scatena R, Bottoni P, Botta G, Martorana GE, Giardina B (2007) The role of mitochondria in pharmacotoxicology: a reevaluation of an old, newly emerging topic. Am J Physiol Cell Physiol 293:C12–C21
Sellin MK, Tate-Boldt E, Kolok AS (2005) Acclimation to Cu in fathead minnows: does age influence the response? Aquat Toxicol 74:97–109
Shukla V, Dhankhar M, Prakash J, Sastry K (2007) Bioaccumulation of Zn, Cu and Cd in Channa punctatus. J Environ Biol 28:395
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:research0034
Vergauwen L, Knapen D, Hagenaars A, Blust R (2013) Hypothermal and hyperthermal acclimation differentially modulate cadmium accumulation and toxicity in the zebrafish. Chemosphere 91:521–529
Vineetha VP, Soumya RS, Raghu KG (2015) Phloretin ameliorates arsenic trioxide induced mitochondrial dysfunction in H9c2 cardiomyoblasts mediated via alterations in membrane permeability and ETC complexes. Eur J Pharmac 754:162–172
Wu C, Zhang D, Kan M, Lv Z, Zhu A, Su Y, Zhou D, Zhang J, Zhang Z, Xu M (2014) The draft genome of the large yellow croaker reveals well-developed innate immunity. Nat. Commun 5
Zheng JL, Luo Z, Chen QL, Liu X, Liu CX, Zhao YH, Gong Y (2011) Effect of waterborne zinc exposure on metal accumulation, enzymatic activities and histology of Synechogobius hasta. Ecotox. Environ. Safe. 74:1864–1873
Zheng JL, Hu W, Luo Z, Zhao YH, Zhu QL, Li XD (2013a) Comparative study on the kinetic behaviour of carnitine palmitoyltransferase I between Javelin goby Synechogobius hasta (carnivorous) and grass carp Ctenopharyngodon idella (herbirovous). Aquac Nutr 19:665–676
Zheng JL, Luo Z, Liu CX, Chen QL, Tan XY, Zhu QL, Gong Y (2013b) Differential effects of acute and chronic zinc (Zn) exposure on hepatic lipid deposition and metabolism in yellow catfish Pelteobagrus fulvidraco. Aquat Toxicol 132-133:173–181
Zheng JL, Luo Z, Zhu QL, Hu W, Zhuo MQ, Pan YX, Song YF, Chen QL (2015) Different effect of dietborne and waterborne Zn exposure on lipid deposition and metabolism in juvenile yellow catfish Pelteobagrus fulvidraco. Aquat Toxicol 159:90–98
Zheng JL, Zhu QL, Wu CW, Zhu AY, Shen B, Zeng L (2016a) Zinc acclimation mitigated high zinc induced oxidative stress by enhancing antioxidant defenses in large yellow croaker Pseudosciaena crocea. Aquat Toxicol 172:21–29
Zheng JL, Zeng L, Xu MY, Shen B, Wu CW (2016b) Different effects of low- and high-dose waterborne zinc on Zn accumulation, ROS levels, oxidative damage and antioxidant responses in the liver of large yellow croaker Pseudosciaena crocea. Fish Physiol Biochem 1–11
Acknowledgments
This work was supported by the Natural Science Foundation of Zhejiang Province (LY15C190009), National Natural Science Foundation of China (Grant No. 41606122), and Public Science and Technology Research Funds Projects of Ocean (201505025).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, JL., Yuan, SS., Shen, B. et al. Organ-specific effects of low-dose zinc pre-exposure on high-dose zinc induced mitochondrial dysfunction in large yellow croaker Pseudosciaena crocea . Fish Physiol Biochem 43, 653–661 (2017). https://doi.org/10.1007/s10695-016-0319-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-016-0319-y