Abstract
This study was undertaken to explore the systemic metabolic strategies of juvenile grass carp (Ctenopharyngodon idellus) to maintain growth when fed with different dietary protein levels. The optimal growth group and two growing discomfort groups were selected through the basic data, to explain the growth difference from appetite regulation and lipid and glucose metabolism perspective. Three experimental diets were formulated with three dietary protein levels at 200.3, 296.1 and 442.9 g kg−1, named P1, P2 and P3, respectively. Juvenile grass carp (initial body weight 12.28 ± 0.14 g) were fed with three diets with 3 replications per dietary treatment in an indoor recirculation system for an 8-week feeding trial. Fish fed with diet P2 dietary group showed significantly higher WG, SGR, FI and PER than other groups. Compared with other groups, mRNA expressions of NPY, Y8a and Y8b in fish fed with P2 significantly down-regulated, while the expressions of CCK and CART in fish fed with P3 significantly down-regulated (P < 0.05). With increasing dietary protein levels, G6Pase, GK, PK and PEPCK were all significantly inhibited (P < 0.05). For lipid metabolism, the mRNA expression of ACC in P1 dietary group was significantly higher than P3 dietary group; besides, LPL expression in P3 group was significantly higher than other two groups (P < 0.05). PPARα expression in P2 was significantly lower than other groups (P < 0.05). These results suggested that grass carp fed with P2 (296.1 g kg−1 protein level) showed highest weight gain, contributed to more balanced nutrient metabolism and appetite regulation. Too high dietary protein (442.9 g kg−1) should be avoided because it induced lowest PER, body lipid and liver lipid, and inhibited glucose and lipid metabolism in juvenile grass carp.



Similar content being viewed by others
Abbreviations
- FI:
-
Feed intake
- WG:
-
Weight gain
- FW:
-
Final body weight
- SGR:
-
Specific growth ratio
- PER:
-
Protein efficiency ratio
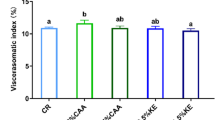
- VSI:
-
Viscerosomatic index
- HSI:
-
Hepatosomatic index
- SR:
-
Survival ratio
- CHO:
-
Cholesterol
- TG:
-
Triglyceride
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- NPY:
-
Neuropeptide Y
- CART:
-
Cocaine- and amphetamine-regulated transcript
- CCK:
-
Cholecystokinin
- ACC:
-
Acetyl-CoA carboxylase
- LPL:
-
Lipoprotein lipase
- PPARα:
-
Peroxisome proliferator-activated receptor type α
- G6Pase:
-
Glucose-6-phosphatase
- GK:
-
Glucokinase
- PK:
-
Pyruvate kinase
- PEPCK:
-
Phosphoenol pyruvate carboxykinase
References
Akpinar Z, Sevgili H, Ozgen T, Demir A, Emre Y (2012) Dietary protein requirement of juvenile shi drum, Umbrina cirrosa (L.). Aquac Res 43:421–429
Alam MS, Watanabe WO, Carroll PM (2008) Dietary protein requirements of Juvenile Black Sea Bass, Centropristis striata. J World Aquac Soc 39:656–663
Aldegunde M, Mancebo M (2006) Effects of neuropeptide Y on food intake and brain biogenic amines in the rainbow trout (Oncorhynchus mykiss). Peptides 27:719–727
Andoh T (2007) Amino acids are more important insulinotropins than glucose in a teleost fish, barfin flounder (Verasper moseri). Gen Comp Endocrinol 151:308–317
Anguiano M, Pohlenz C, Buentello A, Gatlin DM (2013) The effects of prebiotics on the digestive enzymes and gut histomorphology of red drum (Sciaenops ocellatus) and hybrid striped bass (Morone chrysops × M. saxatilis). Br J Nutr 109:623–629
Aoyama T, Fukui K, Takamatsu K, Hashimoto Y, Yamamoto T (2000) Soy protein isolate and its hydrolysate reduce body fat of dietary obese rats and genetically obese mice (yellow KK). Nutrition 16:349–354
Arzel J, Métailler R, Kerleguer C, Delliou HL, Guillaume J (1995) The protein requirement of brown trout (Salmo trutta) fry. Aquaculture 130:67–78
Association of Official Analytical Chemists (AOAC) (1995) Official methods of analysis of official analytical chemists international, 16th edn. AOAC, Arlington
Campos VF, Robaldo RB, Deschamps JC, Fabiana KS, Alan AM, Luis FM, Marcelo O, Luís AS, Tiago C (2012) Neuropeptide Y gene expression around meal time in the Brazilian flounder Paralichthys orbignyanus. J Biosci 37:227–232
Choi YS, Goto S, Ikeda I, Michihiro S (1989) Interaction of dietary protein, cholesterol and age on lipid metabolism of the rat. Br J Nutr 61:531–543
Cowey CB, Walton MJ (1989) Intermediary metabolism. In: Halver JE (ed) Fish nutrition, 2nd edn. Academic Press, New York, pp 259–329
Cowey CB, Adron JW, Brown DA, Shanks AM (1975) Studies on the nutrition of marine flatfish. The metabolism of glucose by plaice (Pleuronectes platessa) and the effect of dietary energy source on protein utilization in plaice. Br J Nutr 33:219–231
Cowey CB, Knox D, Walton MJ, Adron JW (1977) The regulation of gluconeogenesis by diet and insulin in rainbow trout (Salmo gairdneri). Br J Nutr 38:463–470
Cowey CB, Cooke DJ, Matty AJ, Adron JW (1981) Effects of quantity and quality of dietary protein on certain enzyme activities in rainbow trout. J Nutr 111:336–345
Dabrowski K (1977) Protein requirements of grass carp fry (Ctenopharyngodon idella Val.). Aquaculture 12:63–73
Enes P, Panserat S, Kaushik S, Oliva-Teles A (2009) Nutritional regulation of hepatic glucose metabolism in fish. Fish Physiol Biochem 35:519–539
FAO (2013) FAO statistical yearbook 2013. World food and agriculture. Food and Agriculture Organisation for the United Nations, Rome
García-Gallego M, Bazoco J, Suárez MD, Sanz A (1995) Utilization of dietary carbohydrates by fish: a comparative study in eel and trout. Anim Sci 61:427–436
Habte-Tsion HM, Liu B, Ge XP, Xie J, Xu P, Ren MC, Zhou QL, Pan LK, Chen RL (2013) Effects of dietary protein level on growth performance, muscle composition, blood composition, and digestive enzyme activity of Wuchang bream (Megalobrama amblycephala) fry. Israeli J Aquac Bamidgeh 65:1e9
He S, Liang XF, Li L, Sun J, Wen ZY, Cheng XY, Li AX, Cai WJ, He YH, Wang YP, Tao YX, Yuan XC (2015) Transcriptome analysis of food habit transition from carnivory to herbivory in a typical vertebrate herbivore, grass carp Ctenopharyngodon idella. BMC Genom 16:15–29
Jauncey K (1982) The effects of varying dietary protein level on the growth, food conversion, protein utilization and body composition of juvenile tilapias (Sarotherodon mossambicus). Aquaculture 27:43–54
Kamijo M, Kojima K, Maruyama K, Konno N, Motohashi E, Ikegami T, Uchiyama M, Shioda S, Ando H, Matsuda K (2011) Neuropeptide Y in tiger puffer (Takifugu rubripes): distribution, cloning, characterization, and mRNA expression responses toprandial condition. Zool Sci 28:882–890
Kumar P, Jain KK, Munilkumar S, Sahu NP, Pal AK (2013) Effect of feeding normal and low protein diet alternately to Labeo rohita fingerlings on growth performance and biochemical composition. Int J Sci Knowl 2:3–13
Lee SM, Cho SH, Kim KD (2000) Effects of dietary protein and energy levels on growth and body composition of juvenile flounder Paralichthys olivaceus. J World Aquac Soc 31:306–315
Lee SM, Park CH, Bang IC (2002) Dietary protein requirement of young Japanese flounder Paralichthys olivaceus fed isocaloric diets. Fish Sci 68:158–164
Liao CX, Huang ZZ (1987) The dietary protein requirement of grass carp on different growth stage. Freshw Fish 1:1–5
Liu LW, Su JM, Luo YL (2012) Effect of partial replacement of dietary monocalcium phosphate with neutral phytase on growth performance and phosphorus digestibility in gibel carp, Carassius auratus gibelio (Bloch). Aquac Res 43:1404–1413
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Lochmann RT, Phillips H (1994) Dietary protein requirement of juvenile golden shiners (Notemigonus crysoleucas) and goldfish (Carassius auratus) in aquaria. Aquaculture 128:277–285
Lyndon AR, Davidson I, Houlihan DF (1993) Changes in tissue and plasma free amino acid concentrations after feeding in Atlantic cod. Fish Physiol Biochem 10:365–375
Madani S, Prost J, Belleville J (2000) Dietary protein level and origin (casein and highly purified soybean protein) affect hepatic storage, plasma lipid transport, and antioxidative defense status in the rat. Nutrition 16:368–375
Mersmann HJ (1998) Lipoprotein and hormone-sensitive lipases in porcine adipose tissue. J Anim Sci 76:1396–1404
Meyer G, Fracalossi DM (2004) Protein requirement of jundia fingerlings, Rhamdia quelen, at two dietary energy concentrations. Aquaculture 240:331–343
Narnaware YK, Peter RE (2001) Neuropeptide Y gene expression around meal time in the Brazilian flounder Paralichthys orbignyanus. J Biosci 37:227–232
Parazo MM (1990) Effect of dietary protein and energy level on growth, protein utilization and carcass composition of rabbitfish, Siganus guttatus. Aquaculture 86:41–49
Peres H, Oliva-Teles A (1999) Effect of dietary lipid level on growth performance and feed utilization by European sea bass juveniles (Dicentrarchus labrax). Aquaculture 179:325–334
Peters JC, Harper AE (1985) Adaptation of rats to diets containing different levels of protein: effects on food intake, plasma and brain amino acid concentrations and brain neurotransmitter metabolism. J Nutr 115:382–398
Siddiqui TQ, Khan MA (2009) Effects of dietary protein levels on growth, feed utilization, protein retention efficiency and body composition of young Heteropneustes fossilis (Bloch). Fish Physiol Biochem 35:479–488
Silverstein JT, Plisetskaya EM (2000) The effects of NPY and insulin on food intake regulation in fish. Am Zool 40:296–308
Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC (1998) Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 98:2088–2093
Terasawa F, Hirano Y, Wada M, Takita T, Nakamura K, Innami S (1994) Effects of dietary casein and soy-protein on metabolic conversion of eicosapentaenoic acid to docosahexaenoic acid in the liver of rat. J Nutr Sci Vitaminol 40:353–362
Valassi E, Scacchi M, Cavagnini F (2008) Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis 18:158–168
Volkoff H (2006) The role of neuropeptide Y, orexins, cocaine and amphetamine-related transcript, cholecystokinin, amylin and leptin in the regulation of feeding in fish. Comp Biochem Physiol Part A 144:325–331
Volkoff H, Peter RE (2000) Effects of CART peptides on food consumption, feeding and associated behaviors in the gold fish, Carassius auratus: actions on neuropeptide Y and orexin A induced feeding. Brain Res 887:125–133
Volkoff H, Eykelbosh AJ, Peter RE (2003) Role of leptin in the control of feeding of gold fish Carassius auratus: interactions with cholecystokinin, neuropeptide Y and orexin A, and modulation by fasting. Brain Res 972:90–109
Volkoff H, Canosa LF, Unniappan S, Cerda-Reverter JM, Bernier NJ, Kelly SP, Peter RE (2005) Neuropeptides and the control of food intake in fish. Gen Comp Endocrinol 142:3–19
Volkoff H, Unniappan S, Kelly SP (2009) The endocrine regulation of food intake. In: Bernier N, Kraak GVD, Farrell A, Brauner C (eds) Fish physiology, vol 28. Academic Press, Burlington, pp 421–465
Volkoff H, Hoskins LJ, Tuziak SM (2010) Influence of intrinsic signals and environmental cues on the endocrine control of feeding in fish: potential application in aquaculture. Gen Comp Endocrinol 167:352–359
Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, Burden VR, Purnell JQ (2005) A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr 82:41–48
Yamamoto T, Unuma T, Akiyama T (2000) The influence of dietary protein and fat levels on tissue free amino acid levels of fingerling rainbow trout (Oncorhynchus mykiss). Aquaculture 182:353–372
Yang SD, Liou CH, Liu FG (2002) Effects of dietary protein level on growth performance, carcass composition and ammonia excretion in juvenile silver perch (Bidyanus bidyanus). Aquaculture 213:363–372
Zhang J, Zhou F, Wang LL, Shao Q, Xu Z, Xu J (2010) Dietary protein requirement of juvenile black sea bream, Sparus macrocephalus. J World Aquac Soc 41:151–164
Zhao SM, Wang J, Song XL, Zhang X, Ge C, Gao S (2010) Impact of dietary protein on lipid metabolism-related gene expression in porcine adipose tissue. Nutr Metab 7:6–18
Zhou Y, Liang XF, Yuan XC, Li J, He Y, Fang L, Guo XZ, Liu LW, Li B, Shen D (2013) Neuropeptide Y stimulates food intake and regulates metabolism in grass carp, Ctenopharyngodon idellus. Aquaculture 380:52–61
Acknowledgments
This work was financially supported by the National Basic Research Program of China (2014CB138601), the National Natural Science Foundation of China (31272641) and Fundamental Research Funds for the Central Universities (2662015PY041 and 2015QC023).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jiao Li and Liwei Liu have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, J., Liu, L., Liang, XF. et al. Modulation of appetite, lipid and glucose metabolism of juvenile grass carp (Ctenopharyngodon idellus) by different dietary protein levels. Fish Physiol Biochem 43, 297–307 (2017). https://doi.org/10.1007/s10695-016-0287-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-016-0287-2