Abstract
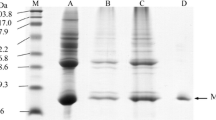
The primary structure of southern bluefin tuna Thunnus maccoyii Mb has been elucidated by molecular cloning techniques. The cDNA of this tuna encoding Mb contained 776 nucleotides, with an open reading frame of 444 nucleotides encoding 147 amino acids. The nucleotide sequence of the coding region was identical to those of other bluefin tunas (T. thynnus and T. orientalis), thus giving the same amino acid sequences. Based on the deduced amino acid sequence, bioinformatic analysis was performed including phylogenic tree, hydropathy plot and homology modeling. In order to investigate the autoxidation profiles, the isolation of Mb was performed from the dark muscle. The water soluble fraction was subjected to ammonium sulfate fractionation (60–90 % saturation) followed by preparative gel electrophoresis. Autoxidation profiles of Mb were delineated at pH 5.6, 6.5 and 7.4 at temperature 37 °C. The autoxidation rate of tuna Mb was slightly higher than that of horse Mb at all pH examined. These results revealed that tuna myoglobin was unstable than that of horse Mb mainly at acidic pH.







Similar content being viewed by others
References
Alderton AL, Faustman C, Liebler DC, Hill DW (2003) Induction of myoglobin redox instability by adduction with 4-hydroxynonenal. Biochemistry 42:4398–4405
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Birnbaum GI, Evans SV, Przybylska M, Rose DR (1994) 1.70 Å resolution structure of myoglobin from yellowfin tuna. An example of a myoglobin lacking the D helix. Acta Cryst D 50:283–289
Brill R (1994) A review of temperature and oxygen tolerance studies of tunas pertinent to fisheries oceanography, movements models and stock assessments. Fish Oceanogr 3:204–216
Brown WD, Dolev A (1963) Autoxidation of beef and tuna oxymyoglobin. J Food Sci 28:207–210
Brown WD, Mebine LB (1969) Autoxidation of oxymyoglobins. J Biol Chem 244:6696–6701
Chen WL, Chow CJ (2001) Studies on the physicochemical properties of milkfish myoglobin. J Food Biochem 25:157–174
Chow CJ (1991) Relationship between the stability and autooxidation rate of myoglobin. J Agric Food Chem 39:22–26
Chow CJ, Ochiai Y, Hashimoto K (1985) Effect of freezing and thawing on the autoxidation of bluefin tuna myoglobin. Bull Jpn Soc Sci Fish 51(12):2073–2078
Chow CJ, Yang JI, Lee PF, Ochiai Y (2009) Effect of acid and alkaline pretreatment on the discoloration rates of dark muscle and myoglobin extract of skinned tilapia fillet during iced storage. Fish Sci 75:1481–1488
Cossins AR, Berenbrink M (2008) Myoglobin’s new clothes. Nature 454:416–417
Faustman C, Cassens RG (1990) The biochemical basis for discoloration in fresh meat: a review. J Muscular Foods 1:217–243
Faustman C, Liebler DC, McClure TD, Sun Q (1999) α, β-Unsaturated aldehydes accelerate oxymyoglobin oxidation. J Agric Food Chem 47:3140–3144
Faustman C, Sun Q, Mancini R, Suman SP (2010) Myoglobin and lipid oxidation interactions: mechanistic bases and control. Meat Sci 86:86–94
Flögel U, Fago A, Rassaf T (2010) Keeping the heart in balance: the functional interactions of myoglobin with nitrogen oxides. J Exp Biol 213:2726–2733
Gutzke D, Trout GR (2002) Temperature and pH dependence of the autoxidation rate of bovine, ovine, porcine, and corvine oxymyoglobin isolated from three different muscles-longissimus dorsi, gluteus medius, and biceps femoris. J Agric Food Chem 50:2673–2678
Hasan MM, Watabe S, Ochiai Y (2012) Structural characterization of carangid fish myoglobins. Fish Physiol Biochem 38:1311–1322
Jaspers RT, Testerink J, Gaspera BD, Chanoine C, Bagowski CP, Laarse WJ (2014) Increased oxidative metabolism and myoglobin expression in zebrafish muscle during chronic hypoxia. Biol Open 3:718–727
Joseph P, Suman SP, Li S, Beach CM, Steinke L, Fontaine M (2010) Characterization of bison (Bison bison) myoglobin. J Meat Sci 84:71–78
Kitahara Y, Matsuoka A, Kobayashi N, Shikama K (1990) Autoxidation of myoglobin from bigeye tuna fish (Thunnus obesus). Biochem Biophys Acta 1038:23–28
Kyte J, Doolittle R (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105–132
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–685
Lee S, Joo ST, Alderton AL, Hill DW, Faustman C (2003) Oxymyoglobin and lipid oxidation in yellowfin tuna (Thunnus albacores) loins. J Food Sci 68:1664–1668
Madden PW, Babcock MJ, Vayda ME, Cashon RE (2004) Structural and kinetic characterization of myoglobins from eurythermal and stenothermal fish species. Comp Biochem Physiol 137B:341–350
Marcinek DJ, Bonaventura J, Wittenberg JB, Block BA (2001) Oxygen affinity and amino acid sequence of myoglobins from endothermic and ectothermic fish. Am J Physiol Regul Integr Comp Physiol 280:R1123–R1133
Nakamura Y, Ando M, Seoka M, Kawasaki K, Tsukamasa Y (2007) Changes of proximate compositions and myoglobin content in the dorsal ordinary muscles of the cultured Pacific bluefin tuna Thunnus orientalis with growth. Fish Sci 74:1155–1159
Nicholas JW, Weber LJ (1989) Comparative oxygen affinity of fish and mammalian myoglobins. J Comp Physiol 159B:205–209
Nurilmala M, Hedeki U, Kaneko G, Ochiai Y (2013) Assessment of commercial quality evaluation of yellowfin tuna thunnus albacares meat based on myoglobin properties. Food Sci Tech Res 19:237–243
Ochiai Y, Ueki N, Watabe S (2009) Effects of point mutations on the structural stability of tuna myoglobins. Comp Biochem Physiol 153B:223–228
Ochiai Y, Watanabe Y, Ozawa H, Ikegami S, Uchida N, Watabe S (2010) Thermal denaturation profiles of tuna myoglobin. Biosci Biotechnol Biochem 74:1673–1679
Phillips SEV, Schoenboen BP (1981) Neutron diffraction reveals oxygen-histidine hydrogen bond in oxymyoglobin. Nature 92:81–82
Schwede T, Kopp J, Guex N, Peitsch MC (2003) SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res 31:3381–3385
Snyder HE, Ayres JC (1961) The autoxidation of crystallized beef myoglobin. J Food Sci 26:469–474
Suman SP, Faustman C, Stamer SL, Liebler DC (2007) Proteomics of lipid oxidation-induced oxidation in porcine and bovine oxymyoglobins. Proteomics 313:628–640
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA 4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tang J, Faustman C, Hoagland TA (2004) Krzywicki revisited: equations for spectrophotometric determination of myoglobin redox forms in aqueous meat extract. J Food Sci 69:C717–C720
Thiansilakul Y, Benjakul S, Richards MP (2011) Isolation, characterisation and stability of myoglobin from eastern little tuna (Euthynnus affinis) dark muscle. Food Chem 124:254–261
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acid Res 22:4673–4680
Trout GR (1989) Variation in myoglobin denaturation and color of cooked beef, pork and turkey meat as influenced by pH, sodium chloride, sodium tripolyphosphate, and cooking temperature. J Food Sci 54:536–540
Ueki N, Ochiai Y (2004) Primary structure and thermostability of bigeye tuna myoglobin in relation to those from other scombridae fish. Fish Sci 70:875–884
Ueki N, Ochiai Y (2006) Effects of amino acid replacement on the structural stability of fish myoglobin. J Biochem 140:649–656
Ueki N, Chow CJ, Ochiai Y (2005) Characterization of bullet tuna myoglobin with reference to thermostability - structure relationship. J Agric Food Chem 53:4968–4975
Wongwichian C, Klomklao S, Panpipat W, Benjakul S, Chaijan M (2015) Interrelationship between myoglobin and lipid oxidations in oxeye scad (Selar boops) muscle during iced storage. Food Chem 174:279–285
Acknowledgments
This work was partly supported by Japan Society for Promotion of Sciences (KAKENHI # 22380015 to Y. O.). The authors would like to thank Prof. Shugo Watabe, Prof. Hideki Ushio and Dr. Hideo Ozawa for their valuable suggestions throughout this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nurilmala, M., Ochiai, Y. Molecular characterization of southern bluefin tuna myoglobin (Thunnus maccoyii). Fish Physiol Biochem 42, 1407–1416 (2016). https://doi.org/10.1007/s10695-016-0228-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-016-0228-0