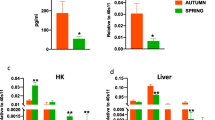
Abstract
The stress response transmitted by the HPA axis is one of the best examples of neuroendocrine–immune interactions that are critical for survival. Analogous to the situation in mammals, the stress response in fish is characterized by the activation of the hypothalamo–pituitary–interrenal axis (HPI). Effects of cortisol on the fish immune system comply with findings in mammals and suggest that the differences in sensitivity to stress will influence the immune response and as a consequence of survival. Therefore, we studied the stress response and its immunity-related effects in four different carp lines (R3, R3xR8, K and R2) that display a differential pathogen susceptibility. Previous studies indicate that R3xR8 and R3 carp are susceptible to bacterial and parasite infection, while R2 and K are relatively resistant to infection. Interestingly, the most striking effect of stress on leukocyte composition and activity was observed in the pathogen-resistant K carp, even though no robust changes in gene expression of stress-involved factors were observed. In contrast, R3 carp showed no spectacular stress-induced changes in their immunological parameters with concurrent significant activation of the HPI axis. Upon stress, the R3 carp showed up-regulation of crf, pomc and gr2 gene expression in the hypothalamus. Furthermore in R3 carp, at all levels of the HPI axis, stress induced the highest up-regulation of il-1β gene expression. Although we are aware of the complexity of the interactions between stress and pathogen susceptibility and of the risk of interpretation based on correlations, it is noteworthy that the fish more susceptible to infection also exhibited the highest response to stress.










Similar content being viewed by others
References
Ainsworth AJ, Dexiang C, Waterstrat PR, Greenway T (1991) Effect of temperature on the immune system of channel catfish (Ictalurus punctatus)—I. Leucocyte distribution and phagocyte function in the anterior kidney at 10 degrees C. Comp Biochem Physiol, A: Comp Physiol 100:907–912
Barnes PJ (2010) Mechanisms and resistance in glucocorticoid control of inflammation. J Steroid Biochem Mol Biol 120:76–85
Barnes PJ, Adcock IM (2009) Glucocorticoid resistance in inflammatory diseases. Lancet 373:1905–1917
Basu N, Kennedy CJ, Iwama GK (2003) The effects of stress on the association between hsp70 and the glucocorticoid receptor in rainbow trout. Comp Biochem Physiol A: Mol Integr Physiol 134:655–663
Bekh V, Irnazarow I, Onara D, Rakus K, Jurecka P, Pilarczyk A (2004) Studies of experimental infection with Aeromonas hydrophila in common carp (Cyprinus carpio L.). In: Siwicki AK, Antychowicz J, Szweda W (eds) Ochrona zdrowia ryb—aktualne problemy. Wyd. IRS., Olsztyn, pp 143–150 (in Polish)
Bury NR, Sturm A (2007) Evolution of the corticosteroid receptor signaling pathway in fish. Gen Comp Endocrinol 153:47–56
Caipang CMA, Berg I, Brinchmann MF, Kiron V (2009) Short-term crowding stress in Atlantic cod, Gadus morhua L. modulates the humoral immune response. Aquaculture 295:110–115
Campbell TW, Ellis CK (2007) Avian and exotic animal hematology and cytology. Blackwell Publishing, Ames
Casadio R, Frigimelica E, Bossù P, Neumann D, Martin MU, Tagliabue A, Boraschi D (2001) Model of interaction of the IL-1 receptor accessory protein IL-1RAcP with the IL-1beta/IL-1R(I) complex. FEBS Lett 499:65–68
Chadzinska M, Savelkoul HF, Verburg-van Kemenade BM (2009) Morphine affects the inflammatory response in carp by impairment of leukocyte migration. Dev Comp Immunol 33:88–96
Dantzer R (2006) Cytokine, sickness behavior, and depression. Neurol Clin 24:441–460
Davis EP, Townsend EL, Gunnar MR, Georgieff MK, Guiang SF, Cifuentes RF, Lussky RC (2004) Effects of prenatal corticosteroid exposure on regulation of stress physiology in healthy premature infants. Psychoneuroendocrinology 29:1028–1036
Dubowski KM (1962) An o-toluidine method for body-fluid glucose determination. Clin Chem 8:215–235
Dunn AJ (2000) Cytokine activation of the HPA axis. Ann N Y Acad Sci 917:608–617
Dunne A, O’Neill LA (2003) The interleukin-1 receptor/toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci Signal 171:re3
Elenkov IJ, Chrousos GP (2006) Stress system—organization, physiology and immunoregulation. Neuro Immuno Modul 13:257–267
Ellis AE (1990) Lysozyme assays. In: Stolen JS, Fletcher TC, Anderson DP, Roberson BS, van Muiswinkel WB (eds) Techniques in fish immunology. SOS Publications, New Jersey, p 101e3
Ellsaesser CF, Clem LW (1987) Cortisol-induced hematologic and immunologic changes in channel catfish (Ictalurus punctatus). Comp Biochem Physiol, A: Comp Physiol 87:405–408
Engelsma MY, Stet RJ, Schipper H, Verburg-van Kemenade BM (2001) Regulation of interleukin 1 beta RNA expression in the common carp, Cyprinus carpio L. Dev Comp Immunol 25:195–203
Engelsma MY, Hougee S, Nap D, Hofenk M, Rombout JH, van Muiswinkel WB, Verburg-van Kemenade BML (2003) Multiple acute temperature stress affects leucocyte populations and antibody responses in common carp, Cyprinus carpio L. Fish Shellfish Immunol 15:397–410
Espelid S, Lokken GB, Steiro K, Bogwald J (1996) Effects of cortisol and stress on the immune system in Atlantic Salmon (Salmo salar L.). Fish Shellfish Immunol 6:95–110
Greenwood AK, Butler PC, White RB, DeMarco U, Pearce D, Fernald RD (2003) Multiple corticosteroid receptors in a teleost fish: distinct sequences, expression patterns, and transcriptional activities. Endocrinology 144:4226–4236
Holland JW, Pottinger TG, Secombes CJ (2002) Recombinant interleukin-1 beta activates the hypothalamic–pituitary–interrenal axis in rainbow trout, Oncorhynchus mykiss. J Endocrinol 175:261–267
Huising MO, Guichelaar T, Hoek C, Verburg-van Kemenade BM, Flik G, Savelkoul HF, Rombout JH (2003) Increased efficacy of immersion vaccination in fish with hyperosmotic pretreatment. Vaccine 21:4178–4193
Huising MO, Metz JR, van Schooten C, Taverne-Thiele AJ, Hermsen T, Verburg-van Kemenade BM, Flik G (2004a) Structural characterisation of a cyprinid (Cyprinus carpio L.) CRH, CRH-BP and CRH-R1, and the role of these proteins in the acute stress response. J Mol Endocrinol 32:627–648
Huising MO, Stet RJ, Savelkoul HF, Verburg-van Kemenade BM (2004b) The molecular evolution of the interleukin-1 family of cytokines; IL-18 in teleost fish. Dev Comp Immunol 28:395–413
Huising MO, Metz JR, De Mazon AF, Verburg-van Kemenade BM, Flik G (2005) Regulation of the stress response in early vertebrates. Ann N Y Acad Sci 1040:345–347
Huising MO, van der Aa LM, Metz JR, de Fátima Mazon A, Verburg-van Kemenade BM, Flik G (2007) Corticotropin-releasing factor (CRF) and CRF-binding protein expression in and release from the head kidney of common carp: evolutionary conservation of the adrenal CRF system. J Endocrinol 193:349–357
Jurecka P, Wiegertjes GF, Rakus KL, Pilarczyk A, Irnazarow I (2009) Genetic resistance of carp (Cyprinus carpio L.) to Trypanoplasma borreli: influence of transferrin polymorphisms. Vet Immunol Immunopathol 127:19–25
Kemenade B, Groeneveld A, Rens B, Rombout J (1994) Characterization of macrophages and neutrophilic granulocytes from the pronephros of carp (Cyprinus carpio). J Exp Biol 187:143–158
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Manuel R, Metz JR, Flik G, Vale WW, Huising MO (2014) Corticotropin-releasing factor-binding protein (CRF-BP) inhibits CRF- and urotensin-I-mediated activation of CRF receptor-1 and -2 in common carp. Gen Comp Endocrinol 202:69–75
Maule AG, Schreck CB (1991) Stress and cortisol treatment changed affinity and number of glucocorticoid receptors in leukocytes and gill of coho salmon. Gen Comp Endocrinol 84:83–93
May MJ, Ghosh S (1998) Signal transduction through NF-kappa B. Immunol Today 19:80–88
Metz JR, Huising MO, Meek J, Taverne-Thiele AJ, Wendelaar Bonga SE, Flik G (2004) Localization, expression and control of adrenocorticotropic hormone in the nucleus preopticus and pituitary gland of common carp (Cyprinus carpio L.). J Endocrinol 182:23–31
Metz JR, Huising MO, Leon K, Verburg-van Kemenade BM, Flik G (2006) Central and peripheral interleukin-1beta and interleukin-1 receptor I expression and their role in the acute stress response of common carp, Cyprinus carpio L. J Endocrinol 191:25–35
Pickering AD, Pottinger TG (1989) Stress responses and disease resistance in salmonid fish: effects of chronic elevation of plasma cortisol. Fish Physiol Biochem 7:253–258
Potter E, Behan DP, Fischer WH, Linton EA, Lowry PJ, Vale WW (1991) Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor-binding proteins. Nature 349:423–426
Rakus KL, Irnazarow I, Adamek M, Palmeira L, Kawana Y, Hirono I, Kondo H, Matras M, Steinhagen D, Flasz B, Brogden G, Vanderplasschen A, Aoki T (2012) Gene expression analysis of common carp (Cyprinus carpio L.) lines during cyprinid herpesvirus 3 infection yields insights into differential immune responses. Dev Comp Immunol 37:65–76
Ruzek MC, Pearce BD, Miller AH, Biron CA (1999) Endogenous glucocorticoids protect against cytokine-mediated lethality during viral infection. J Immunol 162:3527–3533
Sadhu N, Sharma SR, Joseph S, Dube P, Philipose KK (2014) Chronic stress due to high stocking density in open sea cage farming induces variation in biochemical and immunological functions in Asian seabass (Lates calcarifer, Bloch). Fish Physiol Biochem 40:1105–1113
Shintani F, Nakaki T, Kanba S, Sato K, Yagi G, Shiozawa M, Aiso S, Kato R, Asai M (1995) Involvement of interleukin-1 in immobilization stress-induced increase in plasma adrenocorticotropic hormone and in release of hypothalamic monoamines in the rat. J Neurosci 15:1961–1970
Stolte EH, Verburg-van Kemenade BM, Savelkoul HF, Flik G (2006) Evolution of glucocorticoid receptors with different glucocorticoid sensitivity. J Endocrinol 190:17–28
Stolte EH, de Mazon AF, Leon-Koosterziel KM, Jesiak M, Bury NR, Sturm A, Savelkoul HF, Verburg-van Kemenade BM, Flik G (2008a) Corticosteroid receptors involved in stress regulation in common carp, Cyprinus carpio. J Endocrinol 198:403–417
Stolte EH, Nabuurs SB, Bury NR, Sturm A, Flik G, Savelkoul HF, Verburg-van Kemenade BM (2008b) Stress and innate immunity in carp: corticosteroid receptors and pro-inflammatory cytokines. Mol Immunol 46:70–79
Stolte EH, Chadzinska M, Przybylska D, Flik G, Savelkoul HF, Verburg-van Kemenade BM (2009) The immune response differentially regulates Hsp70 and glucocorticoid receptor expression in vitro and in vivo in common carp (Cyprinus carpio L.). Fish Shellfish Immunol 27:9–16
Turnbull AV, Rivier CL (1999) Regulation of the hypothalamic–pituitary–adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev 79:1–71
Umminger BL (1977) Relation of whole blood sugar concentrations in vertebrates to standard metabolic rate. Comp Biochem Physiol, A: Comp Physiol 56:457–460
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625
Westphal NJ, Seasholtz AF (2006) CRH-BP: the regulation and function of a phylogenetically conserved binding protein. Front Biosci 11:1878–1891
Weyts FA, Flik G, Verburg-van Kemenade BM (1998a) Cortisol inhibits apoptosis in carp neutrophilic granulocytes. Dev Comp Immunol 22:563–572
Weyts FA, Flik G, Rombout JH, Verburg-van Kemenade BML (1998b) Cortisol induces apoptosis in activated B cells, not in other lymphoid cells of the common carp, Cyprinus carpio L. Dev Comp Immunol 22:551–562
Yada T, Hyodo S, Schreck CB (2008) Effects of seawater acclimation on mRNA levels of corticosteroid receptor genes in osmoregulatory and immune systems in trout. Gen Comp Endocrinol 156:622–627
Yin Z, Lam TJ, Sin YM (1995) The effects of crowding stress on the non-specific immuneresponse in fancy carp (Cyprinus carpio L.). Fish Shellfish Immunol 5:519–529
Acknowledgments
This work was supported by the Polish National Science Center (Grant No. N N308 572039) and by the Wageningen Institute of Animal Sciences. We are grateful to Marleen Scheer for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pijanowski, L., Jurecka, P., Irnazarow, I. et al. Activity of the hypothalamus–pituitary–interrenal axis (HPI axis) and immune response in carp lines with different susceptibility to disease. Fish Physiol Biochem 41, 1261–1278 (2015). https://doi.org/10.1007/s10695-015-0084-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0084-3