Abstract
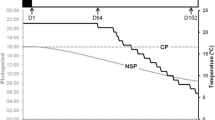
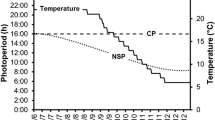
The effect of a constant photoperiod on the inhibition of male and female reproductive cycles was studied in pikeperch Sander lucioperca. Over a 153-day period, batches of pikeperch (2 years, 950 g) breeders were kept under either under natural or artificial photoperiod conditions (24L:0D) (30–35 fish/tank, triplicate) and sampled in late June (start of the photoperiod decrease in natural conditions), late August (start of temperature decrease) and late November (exogenous vitellogenesis) (7–10 fish/tank/sampling date). Morphological parameters, sexual steroids, alkaline-labile phosphate (µg/mL) levels and gamete developmental stages were investigated. Gonado-somatic index (%), developmental stages and sexual steroid levels (17β-estradiol, testosterone and 11-ketotestosterone, ng/mL) in both sexes and oocyte diameter (µm) and plasma alkaline-labile phosphate (µg/mL) in females were lower in response to a continuous lighting (24L:0D). In both sexes, continuous lighting applied in June for 153 days totally inhibited or delayed the onset of the reproductive cycle. In conclusion, photoperiod manipulation can be used to delay the pikeperch reproductive cycle, even if temperature decreases. This is the first report of the inhibitory effect of photoperiod on the onset of the reproductive cycle in pikeperch.








Similar content being viewed by others
References
Abdulfatah A, Fontaine P, Kestemont P, Gardeur JN, Marie M (2011) Effects of photothermal kinetics and amplitude of photoperiod decrease on the induction of the reproduction cycle in female Eurasian perch Perca fluviatilis. Aquaculture 322–323:169–176
Abdulfatah A, Fontaine P, Kestemont P, Milla S, Marie M (2013) Effects of the thermal threshold and the timing of temperature reduction on the initiation and course of oocyte development in cultured female of Eurasian perch Perca fluviatilis. Aquaculture 376–379:90–96
Almeida FF, Taranger GL, Norberg B, Karlsen Ø, Bogerd J, Schulz RW (2009) Photoperiod-modulated testis maturation in Atlantic cod (Gadus morhua, L.). Biol Reprod 80(4):631–640
Amer MA, Miura T, Miura C, Yamauchi K (2001) Involvement of sex steroid hormones in the early stages of spermatogenesis in Japanese huchen (Hucho perryi). Biol Reprod 65(4):1057–1066
Babin PJ, Carnevali O, Lubzens E, Schneider WJ (2007) Molecular aspects of oocyte vitellogenesis in fish. In: Babin PJ, Cerdá J, Lubzens E (eds) The fish oocyte: from basic studies to biotechnological applications. Springer, The Netherlands, pp 39–76
Billard R (1986) Spermatogenesis and spermatology of some teleost fish species. Reprod Nutr Dev 26:877–920
Billard R, Fostier A, Weil C, Breton B (1982) Endocrine control of spermatogenesis in teleost fish. Can J Fish Aquat Sci 39:65–79
Borg B (1994) Androgens in teleost fish. Comp Biochem Physiol 109C:219–245
Bromage N, Porter M, Randall C (2001) The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquaculture 197:63–98
Carrillo M, Zanuy S, Prat F, Serrano R, Bromage N (1993) Environmental and hormonal control of reproduction in sea bass. In: Bromage N, Donaldson EM, Carrillo M, Zanuy S, Planas J (eds) Recent advances in aquaculture IV. Blackwell Science, Oxford, pp 43–54
Carrillo M, Zanuy S, Prat F, Cerda J, Ramos J, Mañanós E, Bromage N (1995) Sea bass (Dicentrarchus labrax). In: Bromage NR, Roberts RJ (eds) Broodstock management and egg and larval quality. Blackwell Science, Oxford, pp 138–168
Davie A, Porter MJ, Bromage NR, Migaud H (2007a) The role of seasonally altering photoperiod in regulating physiology in Atlantic cod (Gadus morhua). Part I. Sexual maturation. Can J Fish Aquat Sci 64(1):84–97
Davie A, Mazorra de Quero C, Bromage NR, Treasurer J, Migaud H (2007b) Inhibition of sexual maturation in tank reared haddock (Melanogrammus aeglefinus) through the use of constant light photoperiods. Aquaculture 270(1):379–389
Divers SL, McQuillan HJ, Matsubara H, Todo T, Lokman PM (2010) Effects of reproductive stage and 11-ketotestosterone on LPL mRNA levels in the ovary of the shortfinned eel. J Lipid Res 51(11):3250–3258
FAO© (2013) Cultured Aquatic Species Information Programme. Sander lucioperca. Cultured Aquatic Species Information Programme. Text by Zakęś Z, in: FAO Fisheries and Aquaculture Department. http://www.fao.org/fishery/culturedspecies/Sander_lucioperca/en. Accessed 02 December 2013
Fontaine P (2009) Développement de la pisciculture continentale européenne et domestication de nouvelles espèces. Cah Agric 2–3:144–146 (in French)
Fostier A, Jalabert B, Billard R, Breton B (1983) The gonadal steroids. In: Hoar WS, Randall DJ, Donaldson EM (eds) Fish physiology IX A. Academic Press, New York, pp 277–345
Gagné F, Blaise C (2000) Organic Alkali-Labile Phosphates in biological materials: a generic assay to detect vitellogenin in biological tissues. Environ Toxicol 15:243–247
Hansen T, Karlsen Ø, Taranger GL, Hemre G-H, Holm JC, Kjesbu OS (2001) Growth, gonadal development and spawning time of Atlantic cod (Gadus morhua) reared under diffrent photoperiods. Aquaculture 203:51–67
Henderson BA, Wong JL, Nepszy SJ (1996) Reproduction of Walleye in lake Erie: allocation of energy. Can J Fish Aquat Sci 53:127–133
Hermelink B, Wuertz S, Trubiroha A, Rennert B, Kloas W, Schulz C (2011) Influence of temperature on puberty and maturation of pikeperch, Sander lucioperca. Gen Comp Endocrinol 172:282–292
Hermelink B, Wuertz A, Rennert B, Kloas W, Schulz C (2013) Temperature control of pikeperch (Sander lucioperca) maturation in recirculating aquaculture systems—induction of puberty and course of gametogenesis. Aquaculture 400–401:36–45
Hildahl J, Taranger GL, Norberg B, Haug TM, Weltzien FA (2013) Differential regulation of GnRH ligand and receptor genes in the brain and pituitary of Atlantic cod exposed to different photoperiod. Gen Comp Endocrinol 180:7–14
Imsland AK, Folkvord A, Jónsdóttir ÓD, Stefansson SO (1997) Effects of exposure to extended photoperiods during the first winter on long-term growth and age at first maturity in turbot (Scophthalmus maximus). Aquaculture 159(1):125–141
Kestemont P, Mélard C (2000) Aquaculture. In: Craig JF (ed) Percid fishes systemics, ecology and exploitation. Blackwell Science, Oxford, pp 191–224
Kobayashi M, Aida K, Stacey NE (1991) Induction of testis development by implantation of 11-ketotestosterone in female goldfish. Zool Sci 8(2):389–393
Langeron M (1942) Précis de Microscopie: Technique-Expérimentation-Diagnostic. Masson et Cie, Paris. 1339 pp (in French)
Lappalainen J, Dorner H, Wysujack K (2003) Reproduction biology of pikeperch (Sander lucioperca L.)—a review. Ecol Freshw Fish 12:95–106
Lokman P, Harris B, Kusakabe M, Kime DE, Schulz RW, Adachi S, Young G (2002) 11-Oxygenated androgens in female teleosts: prevalence, abundance, and life history implications. Gen Comp Endocrinol 129(1):1–12
Lokman PM, George KA, Divers SL, Algie M, Young G (2007) 11-Ketotestosterone and IGF-I increase the size of previtellogenic oocytes from shortfinned eel, Anguilla australis, in vitro. Reproduction 133:955–967
Lubzens E, Cerdà J, Young G, Bobe J (2010) Oogenesis in teleost fish: how fish eggs are formed. Gen Comp Endocrinol 165:367–389
M’Hetli M, Ben Khemis I, Hamza N, Turki B, Turki O (2011) Allometric growth and reproductive biology traits of pikeperch Sander lucioperca at the southern edge of its range. J Fish Biol 78:567–579
Malison JA, Procarione LS, Kayes TB, Hansen JF, Held JA (1998) Induction of out-of-season spawning in walleye (Stizostedion vitreum). Aquaculture 163:151–161
Melamed P, Rosenfeld H, Elizur A, Yaron Z (1998) Endocrine regulation of gonadotropin and growth hormone gene transcription in fish. Comp Biochem Physiol, C: Comp Pharmacol Toxicol 119(3):325–338
Migaud H, Fontaine P, Sulistyo I, Kestemont P, Gardeur JN (2002) Induction of out-of-season spawning in Eurasian perch Perca fluviatilis. Effects of rates of cooling and cooling durations on female gametogenesis and spawning. Aquaculture 205:253–267
Migaud H, Mandiki R, Gardeur JN, Kestemont P, Bromage N, Fontaine P (2003) Influence of photoperiod regimes on the Eurasian perch gonadogenesis and spawning. Fish Physiol Biochem 28:395–397
Migaud H, Gardeur JN, Kestemont P, Fontaine P (2004a) Off-season spawning of Eurasian perch Perca fluviatilis. Aquac Int 12:87–102
Migaud H, Fontaine P, Kestemont P, Wang N, Brun-Bellut J (2004b) Influence of photoperiod on the onset of gonadogenesis in Eurasian perch Perca fluviatilis. Aquaculture 241:561–574
Migaud H, Wang N, Gardeur JN, Fontaine P (2006) Influence of photoperiod on reproductive performances in Eurasian perch Perca fluviatilis. Aquaculture 252:385–393
Migaud H, Davie A, Taylor JF (2010) Current knowledge on the photoneuroendocrine regulation of reproduction in temperate fish species. J Fish Biol 76:27–68
Milla S, Mandiki SNM, Hubermont P, Rougeot C, Mélard C, Kestemont P (2009) Ovarian steroidogenesis inhibition by constant photothermal conditions is caused by a lack of gonadotropin stimulation in Eurasian perch. Gen Comp Endocrinol 163(3):242–250
Miura C, Miura T (2011) Analysis of spermatogenesis using an eel model. Aqua-BioSci Monogr 4(4):105–129
Miura T, Yamauchi K, Takahashi H, Nagahama Y (1991) Hormonal induction of all stages of spermatogenesis in vitro in the male Japanese eel (Anguilla japonica). Proc Natl Acad Sci USA 88:5774–5778
Mueller-Belecke A, Zienert S (2008) Out-of-season spawning of pikeperch (Sander lucicoperca L.) without the need for hormonal treatments. Aquacult. Res. 39:1279-1285
Mylonas CC, Fostier A, Zanuy S (2010) Broodstock management and hormonal manipulations of fish reproduction. Gen Comp Endocrinol 165:516–534
Nagahama Y (1994) Endocrine regulation of gametogenesis. Fish Int J Dev Biol 38:217–229
Poulet N (2004) Le sandre (Sander lucioperca (L.)): biologie, comportement et dynamique des populations en Camargue (Bouches du Rhône, France). Thesis University of Toulouse III (in French)
Pourhosein Sarameh S, Falahatkar B, Takami GA, Efatpanah I (2012) Effects of different photoperiods and handling stress on spawning and reproductive performance of pikeperch Sander lucioperca. Anim Reprod Sci 132(3):213–222
Rad F, Bozaoğlu S, Ergene Gözükara S, Karahan A, Kurt G (2006) Effects of different long-day photoperiods on somatic growth and gonadal development in Nile tilapia (Oreochromis niloticus L.). Aquaculture 255(1):292–300
Remacle C (1976) Actions hormonales sur les cellules germinales males de Carassius auratus L. en culture organotypique. Gen Comp Endocrinol 29:480–491 (in French)
Rinchard J, Kestemont P (1996) Comparative study of reproductive biology in single and multiple spawner cyprinid fish: I. Morphological and histological features. J Fish Biol 49:883–894
Rohr DH, Lokman PM, Davie PS, Young G (2001) 11-Ketotestosterone induces silvering-related changes in immature female short-finned eels, Anguilla australis. Comp Biochem Physiol A: Mol Integr Physiol 130:701–714
Schlumberger O, Proteau JP (1996) Reproduction of pike-perch (Stizostedion lucioperca) in captivity. J Appl Ichthyol 12:149–152
Schneider F, Poehland R (2009) Endocrinology of reproduction. In: Jamieson BG (ed) Reproductive biology and phylogeny of fishes (Agnathans and bony fishes). Reproductive Biology and Phylogeny Series, Science Publishers, pp 54–93
Schulz RW, de França LR, Lareye JJ, Le Gac F, Chiarini-Garcia H, Nobrega RH, Miura T (2010) Spermatogenesis in fish. Gen Comp Endocrinol 165:390–411
Shewmon LN, Godwin JR, Murashige RS, Daniels HV (2007) Environmental manipulation of growth and sexual maturation in yellow perch Perca flavescens. J World Aquac Soc 38:383–394
Sumpter JP (1990) General concepts of seasonal reproduction. In: Munro AD, Scott AP, Lam TJ (eds) Reproductive Seasonality in Teleosts: Environmental Influences. CRC Press, Boca Raton, pp 13–31
Taranger GL, Carillo M, Schulz RW, Fontaine P, Zanuy S, Felip A, Weltzien FA, Dufour S, Karlsen Ǿ, Norberg B, Andersson E, Hansen T (2010) Control of puberty in farmed fish. Gen Comp Endocrinol 165:483–515
Teletchea F, Fontaine P (2014) Levels of domestication in fish: implications for the sustainable future of aquaculture. Fish Fish 15:181–195
Turner CL (1919) The seasonal cycle in the spermaty of the perch. J Morphol 32:681–711
Wallace RA, Selman K (1981) Cellular dynamic aspects of oocyte growth in teleosts. Am Zool 21:325–343
Wang N, Teletchea F, Kestemont P, Milla S, Fontaine P (2010) Photothermal control of the reproductive cycle in temperate fishes. Rev Aquac 2:209–222
Zakes Z, Demska-Zakes K (2009) Controlled reproduction of pikeperch Sander lucioperca (L.). A review. Arch Pol Fish 17:153–170
Acknowledgments
We would like to thank all members of the AFPA Research Unit for their active collaboration in this study. We also like to thank Valérie Legué and Frédéric Guinet from UMR INRA/Lorraine University “Interactions arbres-microorganismes” (IAM) and Maryline Harroué from UMR INRA/AgroParis Tech “Laboratoire d’étude des ressources forêt-bois” (Lerfob) for the provision of the necessary equipment to achieve the histological analysis (Technical Platform of functional ecogenomics, INRA Champenoux, France) and Daniel Van Vlaender from Facultés Universitaires Notre-Dame de la Paix (FUNDP), Namur, Belgium for his help with histological and ALP protocols. This study was partly funded by the Company ASIALOR and the Tunisian Ministry of Higher Education and Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ben Ammar, I., Teletchea, F., Milla, S. et al. Continuous lighting inhibits the onset of reproductive cycle in pikeperch males and females. Fish Physiol Biochem 41, 345–356 (2015). https://doi.org/10.1007/s10695-014-9987-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-014-9987-7