Abstract
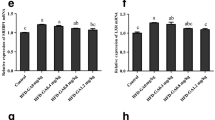
This study investigated the effects of clofibrate treatment on blood lipids, hepatic enzyme activities and relative expression of genes involved in lipid metabolism of grass carp fed with high non-protein energy diets. For that purpose, five diets were formulated: a commercial-like diet (Control), a high-carbohydrate diet (HC), a high-fat diet (HF) and two diets identical to the HC and HF diets, but supplemented with 1.25 g kg−1 clofibrate (HC + Clo and HF + Clo diets). Grass carp fed the HC and HF diet exhibited increases in blood lipids and body fat compared with the control group after 4 weeks. In the clofibrate treatment groups, there was a marked decrease in triacylglycerol and cholesterol concentrations of plasma, and total lipids of the whole body, mesentery adipose tissue and liver tissue. Fish treated with clofibrate exhibited increased hepatic acyl-CoA oxidase activity, but did not show any changes in carnitine palmitoyltransferase (CPT) I activity compared with HC and HF diets without clofibrate. Clofibrate treatment had no effect on peroxisome proliferator-activated receptor alpha and CPT I mRNA expression. However, there was an increase in lipoprotein lipase expression in the clofibrate-treated groups. In addition, the relative mRNA expression levels of hepatic de novo lipogenic enzymes (fatty acid synthetase and acetyl coenzyme-A carboxylase) were significantly higher in the fish fed the HC diet than those of other groups, and clofibrate inhibited this increase. These results suggest that clofibrate has the hypolipidaemic effects and affects lipid metabolism in grass carp.


Similar content being viewed by others
References
Albalat A, Sánchez-Gurmaches J, Gutiérrez J, Navarro I (2006) Regulation of lipoprotein lipase activity in rainbow trout (Oncorhynchus mykiss) tissues. Gen Com Endocrinol 146:226–235
Bardot O, Clemencet MC, Malki MC, Latruffe N (1995) Delayed effects of ciprofibrate on rat liver peroxisomal properties and proto-oncogene expression. Biochem Pharmacol 50:1001–1006
Berthou L, Duverger N, Emmanuel F, Langouët S, Auwerx J, Guillouzo A, Fruchart JC, Rubin E, Denèfle P, Staels B (1996) Opposite regulation of human versus mouse apolipoprotein AI by fibrates in human apolipoprotein AI transgenic mice. J Clin Invest 97:2408
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bremer J (1981) The effect of fasting on the activity of liver carnitine palmitoyltransferase and its inhibition by malonyl-CoA. BBA-Lipid Lipid Met 665:628–631
Chaiyapechara S, Casten MT, Hardy RW, Dong FW (2003) Fish performance, fillet characteristics, and health assessment index of rainbow trout (Oncorhynchus mykiss) fed diets containing adequate and high concentrations of lipid and vitamin E. Aquaculture 219:715–738
Cheng HL, Meng XP, Sun SP, Shi XY, Peng YX, Dong ZG, Shen X (2009) Cloning and expression analysis of a cDNA encoding lipoprotein lipase from the liver of adult grass carp (Ctenopharyngodon idella). Aquac Res 40:1838–1848
Clapham J, Arch J (2007) Thermogenic and metabolic antiobesity drugs: rationale and opportunities. Diabetes Obes Metab 9:259–275
Craig SR, Washburn BS, Gatlin DM (1999) Effects of dietary lipids on body composition and liver function in juvenile red drum, Sciaenops ocellatus. Fish Physiol Biochem 21:249–255
Després JP (2001) Increasing high-density lipoprotein cholesterol: an update on fenofibrate. Am J Cardiol 88:30–36
Desvergne B, Wahli W (1999) Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 20:649–688
Dias J, Alvarez M, Diez A, Arzel J, Corraze G, Bautista J, Kaushik S (1998) Regulation of hepatic lipogenesis by dietary protein/energy in juvenile European seabass (Dicentrarchus labrax). Aquaculture 161:169–186
Du ZY, Demizieux L, Degrace P, Gresti J, Moindrot B, Liu YJ, Tian LX, Cao JM, Clouet P (2004) Alteration of 20:5n−3 and 22:6n−3 fat contents and liver peroxisomal activities in fenofibrate-treated rainbow trout. Lipids 39:849–855
Du ZY, Liu YJ, Tian LX, Wang JT, Wang Y, Liang GY (2005) Effect of dietary lipid level on growth, feed utilization and body composition by juvenile grass carp (Ctenopharyngodon idella). Aquac Nutr 11:139–146
Du ZY, Clouet P, Degrace P, Zheng WH, Frøyland L, Tian LX, Liu YJ (2008) Hypolipidaemic effects of fenofibrate and fasting in the herbivorous grass carp (Ctenopharyngodon idella) fed a high-fat diet. Br J Nutr 100:1200–1212
Frederiksen KS, Wulff EM, Sauerberg P, Mogensen JP, Jeppesen L, Fleckner J (2004) Prediction of PPAR-α ligand-mediated physiological changes using gene expression profiles. J Lipid Res 45:592–601
Gao W, Liu YJ, Tian LX, Mai KS, Liang GY, Yang HJ, Huai MY, Luo WJ (2010) Effect of dietary carbohydrate-to-lipid ratios on growth performance, body composition, nutrient utilization and hepatic enzymes activities of herbivorous grass carp (Ctenopharyngodon idella). Aquac Nutr 16:327–333
Gélineau A, Corraze G, Boujard T, Larroquet L, Kaushik S (2001) Relation between dietary lipid level and voluntary feed intake, growth, nutrient gain, lipid deposition and hepatic lipogenesis in rainbow trout. Reprod Nutr Dev 41:487–504
Guo Q, Wang PR, Milot DP, Ippolito MC, Hernandez M, Burton CA, Wright SD, Chao YS (2001) Regulation of lipid metabolism and gene expression by fenofibrate in hamsters. BBA-Mol Cell Biol 1533:220–232
Guo X, Liang XF, Fang L, Yuan X, Zhou Y, Zhang J, Li B (2013) Effects of dietary non-protein energy source levels on growth performance, body composition and lipid metabolism in herbivorous grass carp (Ctenopharyngodon idella Val.). Aquac Res. doi:10.1111/are.12275
Hawkins JM, Jones WE, Bonner FW, Gibson GG (1987) The effect of peroxisome prouferators on microsomal. Peroxisomal, and mitochondrial enzyme activities in the liver and kidney. Drug Metab Rev 18:441–515
Holden P, Tugwood J (1999) Peroxisome proliferator-activated receptor alpha: role in rodent liver cancer and species differences. J Mol Endocrinol 22:1–8
Ibabe A, Bilbao E, Cajaraville MP (2005) Expression of peroxisome proliferator-activated receptors in zebrafish (Danio rerio) depending on gender and developmental stage. Histochem Cell Biol 123:75–87
Katsutani N, Sekido T, Aoki T, Sagami F (2000) Hepatic drug metabolizing enzymes induced by clofibrate in rasH2 mice. Toxicol Lett 115:223–229
Kersten S, Wahli W (2000) Peroxisome proliferator activated receptor agonists. New approaches to drug development. Springer, Berlin, pp 141–151
Kondo H, Misaki R, Gelman L, Watabe S (2007) Ligand-dependent transcriptional activities of four torafugu pufferfish Takifugu rubripes peroxisome proliferator-activated receptors. Gen Comp Endocrinol 154:120–127
Kusunoki J, Kanatani A, Moller DE (2006) Modulation of fatty acid metabolism as a potential approach to the treatment of obesity and the metabolic syndrome. Endocrine 29:91–100
Lazarow PB (1977) Three hypolipidemic drugs increase hepatic palmitoyl-coenzyme A oxidation in the rat. Science 197:580–581
Leng X, Wu X, Tian J, Li X, Guan L, Weng D (2012) Molecular cloning of fatty acid synthase from grass carp (Ctenopharyngodon idella) and the regulation of its expression by dietary fat level. Aquac Nutr 18:551–558
Lindberg A, Olivecrona G (2002) Lipoprotein lipase from rainbow trout differs in several respects from the enzyme in mammals. Gene 292:213–223
Luci S, Giemsa B, Kluge H, Eder K (2007) Clofibrate causes an upregulation of PPAR-α target genes but does not alter expression of SREBP target genes in liver and adipose tissue of pigs. Am J Physiol Regul Integr Comp Physiol 293:R70–R77
Mandard S, Müller M, Kersten S (2004) Peroxisome proliferator-activated receptor α target genes. Cell Mol Life Sci 61:393–416
Mesia-Vela S, Sanchez RI, Roberts KG, Reuhl KR, Conney AH, Kauffman FC (2008) Dietary clofibrate stimulates the formation and size of estradiol-induced breast tumors in female August-Copenhagen Irish (ACI) rats. Toxicology 246:63–72
Mimeault C, Woodhouse A, Miao XS, Metcalfe C, Moon T, Trudeau V (2005) The human lipid regulator, gemfibrozil bioconcentrates and reduces testosterone in the goldfish, Carassius auratus. Aquat Toxicol 73:44–54
Mimeault C, Trudeau V, Moon T (2006) Waterborne gemfibrozil challenges the hepatic antioxidant defense system and down-regulates peroxisome proliferator-activated receptor beta (PPARβ) mRNA levels in male goldfish (Carassius auratus). Toxicology 228:140–150
Muzio G, Maggiora M, Trombetta A, Martinasso G, Reffo P, Colombatto S, Canuto RA (2003) Mechanisms involved in growth inhibition induced by clofibrate in hepatoma cells. Toxicology 187:149–159
Nunes B, Carvalho F, Guilhermino L (2004) Acute and chronic effects of clofibrate and clofibric acid on the enzymes acetylcholinesterase, lactate dehydrogenase and catalase of the mosquitofish, Gambusia holbrooki. Chemosphere 57:1581–1589
Prindiville JS, Mennigen JA, Zamora JM, Moon TW, Weber JM (2011) The fibrate drug gemfibrozil disrupts lipoprotein metabolism in rainbow trout. Toxicol Appl Pharm 251:201–208
Raldúa D, André M, Babin PJ (2008) Clofibrate and gemfibrozil induce an embryonic malabsorption syndrome in zebrafish. Toxicol Appl Pharm 228:301–314
Roberts RA, James NH, Woodyatt NJ, Macdonald N, Tugwood JD (1998) Evidence for the suppression of apoptosis by the peroxisome proliferator activated receptor alpha (PPAR alpha). Carcinogenesis 19:43–48
Roglans N, Bellido A, Rodriguez C, Cabrero A, Novell F, Ros E, Zambón D, Laguna JC (2002) Fibrate treatment does not modify the expression of acyl coenzyme A oxidase in human liver. Clin Pharmacol Ther 72:692–701
Rørvik KA, Alne H, Gaarder M, Ruyter B, Måseide N, Jakobsen J, Berge R, Sigholt T, Thomassen M (2007) Does the capacity for energy utilization affect the survival of post-smolt Atlantic salmon, Salmo salar L., during natural outbreaks of infectious pancreatic necrosis? J Fish Dis 30:399–409
Rueda-Jasso R, Conceicao LEC, Dias J, De Coen W, Gomes E, Rees JF, Soares F, Dinis MT, Sorgeloos P (2004) Effect of dietary non-protein energy levels on condition and oxidative status of Senegalese sole (Solea senegalensis) juveniles. Aquaculture 231:417–433
Ruyter B, Andersen Ø, Dehli A, Östlund Farrants AK, Gjøen T, Thomassen MS (1997) Peroxisome proliferator activated receptors in Atlantic salmon (Salmo salar): effects on PPAR transcription and acyl-CoA oxidase activity in hepatocytes by peroxisome proliferators and fatty acids. BBA-Lipid Lipid Met 1348:331–338
Scarano LJ, Calabrese EJ, Kostecki PT, Baldwin LA, Leonard DA (1994) Evaluation of a rodent peroxisome proliferator in two species of freshwater fish: rainbow trout (Onchorynchus mykiss) and Japanese medaka (Oryzias latipes). Ecotoxicol Environ Saf 29:13–19
Schoonjans K, Staels B, Auwerx J (1996a) The peroxisome proliferator activated receptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation. BBA-Lipid Lipid Met 1302:93–109
Schoonjans K, Staels B, Auwerx J (1996b) Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res 37:907–925
Shimeno S, Kheyyali D, Shikata T (1995) Metabolic response to dietary lipid to protein ratios in common carp. Fish Sci 61:977–980
Skolness SY, Durhan EJ, Jensen KM, Kahl MD, Makynen EA, Villeneuve DL, Ankley GT (2012) Effects of gemfibrozil on lipid metabolism, steroidogenesis, and reproduction in the fathead minnow (Pimephales promelas). Environ Toxicol Chem 31:2615–2624
Small GM, Burdette K, Connock MJ (1985) A sensitive spectrophotometric assay for peroxisomal acy-CoA oxidase. Biochem J 227:205–210
Staels B, van Tol A, Andreu T, Auwerx J (1992) Fibrates influence the expression of genes involved in lipoprotein metabolism in a tissue-selective manner in the rat. Arterioscler Thromb Vas 12:286–294
Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC (1998) Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 98:2088–2093
Thumelin S, Esser V, Charvy D, Kolodziej M, Zammit V, McGarry D, Girard J, Pegorier J (1994) Expression of liver carnitine palmitoyltransferase I and II genes during development in the rat. Biochem J 300:583–587
Tian Q, Grzemski FA, Panagiotopoulos S, Ahokas JT (2006) Peroxisome proliferator-activated receptor alpha agonist, clofibrate, has profound influence on myocardial fatty acid composition. Chem-Biol Interact 160:241–251
Tian LX, Liu YJ, Yang HJ, Liang GY, Niu J (2012) Effects of different dietary wheat starch levels on growth, feed efficiency and digestibility in grass carp (Ctenopharyngodon idella). Aquac Int 20:283–293
Tsoko M, Beauseigneur F, Gresti J, Demarquoy J, Clouet P (1998) Hypolipidaemic effects of fenofibrate are not altered by mildronate-mediated normalization of carnitine concentration in rat liver. Biochimie 80:943–948
Venkatachalam AB, Lall SP, Denovan-Wright EM, Wright JM (2012) Tissue-specific differential induction of duplicated fatty acid-binding protein genes by the peroxisome proliferator, clofibrate, in zebrafish (Danio rerio). BMC Evol Biol 12:112
Vu-Dac N, Chopin-Delannoy S, Gervois P, Bonnelye E, Martin G, Fruchart JC, Laudet V, Staels B (1998) The nuclear receptors peroxisome proliferator-activated receptor α and Rev-erbα mediate the species-specific regulation of apolipoprotein AI expression by fibrates. J Biol Chem 273:25713–25720
Weston A, Caminada D, Galicia H, Fent K (2009) Effects of lipid-lowering pharmaceuticals bezafibrate and clofibric acid on lipid metabolism in fathead minnow (Pimephales promelas). Environ Toxicol Chem 28:2648–2655
Willson TM, Wahli W (1997) Peroxisome proliferator-activated receptor agonists. Curr Opin Chem Biol 1:235–241
Yaacob NS, Norazmi MN, Gibson GG, Kass GE (2001) The transcription of the peroxisome proliferator-activated receptor α gene is regulated by protein kinase C. Toxicol Lett 125:133–141
Yoon M (2009) The role of PPARα in lipid metabolism and obesity: focusing on the effects of estrogen on PPARα actions. Pharmacol Res 60:151–159
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (31272641 and 31172420), the National Basic Research Program of China (2014CB138601), the Special Fund for Agro-Scientific Research in the Public Interest of China (201003020) and the Fundamental Research Funds for the Central Universities (2011PY030).
Conflict of interest
The authors declare that they do not have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, X., Liang, XF., Fang, L. et al. Effects of lipid-lowering pharmaceutical clofibrate on lipid and lipoprotein metabolism of grass carp (Ctenopharyngodon idellal Val.) fed with the high non-protein energy diets. Fish Physiol Biochem 41, 331–343 (2015). https://doi.org/10.1007/s10695-014-9986-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-014-9986-8