Abstract
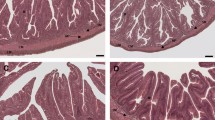
In the present study, the protective effect of nucleotides over damages induced by the consumption of a diet containing a high amount of vegetable ingredients (560 g kg−1) in the intestinal epithelia of the meagre (Argyrosomus regius) was assessed by assays performed with an Ussing-type chamber. Two experimental feeds were prepared including or not a commercial mixture of nucleotides (1 g kg−1). Nucleotides significantly enhanced fish growth during the experiment. On the other hand, differences in the integrity and functionality of intestinal epithelia were evidenced by a change in the polarity of intestinal trans-epithelial potential. Samples of fish fed on the control diet showed a preferentially secretory short-circuit current, while those of fish receiving the nucleotide-supplemented diet showed a significantly lower and preferentially absorptive negative current. It is concluded that alterations of intestinal physiology juvenile meagre resulting from the intake of high amounts of plant ingredients could be minimized by nucleotide supplementation.

Similar content being viewed by others
References
Baumgart DC, Dignass AU (2002) Intestinal barrier function. Curr Opin Clin Nutr Metab Care 5:685–694
Berkes J, Viswanathan VK, Savkovic SD (2003) Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut 52:439–451
Bertelsen LS, Paesold G, Marcus SL, Finlay BB, Eckmann L, Barrett KE (2004) Modulation of chloride secretory responses and barrier function of intestinal epithelial cells by the Salmonella effector protein SigD. Am J Physiol Cell Physiol 287:C939–C948
Borda E, Martinez-Puig D, Cordoba X (2003) A balanced nucleotide supply makes sense. Feed Mix 11:24–26
Buddington RK, Krogdahl A, Bakke-McKellep AM (1997) The intestines of carnivorous fish: structure and functions and the relations with diet. Acta Physiol Scand Suppl 638:67–80
Bueno J, Torres M, Almendros A, Carmona R, Nunez MC, Rios A, Gil A (1994) Effect of dietary nucleotides on small intestinal repair after diarrhea. Histological and ultrastructural changes. Gut 35:926–933
Burrells C, William PD, Southage PJ, Wadsworth SL (2001) Dietary nucleotides: a novel supplement in fish feeds 2. Effects on vaccination, salt water transfer, growth rate and physiology of Atlantic salmon. Aquaculture 199:171–184
Clarke LL (2009) A guide to Ussing chamber studies of mouse intestine. Am J Physiol Gastrointest Liver Physiol 296:G1151–G1166
Estévez A, Treviño L, Kotzamanis Y, Karacostas I, Tort L, Gisbert E (2011) Effects of different levels of plant protein on the on growing of meagre (Argyrosomus regius) juvenile low temperatures. Aquac Nutr 17(2):338–341
Faggio C, Torre A, Lando G, Sabatino G, Trischitta F (2011) Carbonate precipitates and bicarbonate secretion in the intestine of sea bass, Dicentrarchus labrax. J Comp Physiol B181:517–525
Francis G, Makkar HPS, Becker K (2001) Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 199:197–227
Fuentes J, Power DM, Canario AVM (2010) Parathyroid hormone-related protein-stanniocalcin antagonism in regulation of bicarbonate secretion and calcium precipitation in a marine fish intestine. Am Physiol Soc Am J Physiol Regul Integr Comp Physiol 299:R150–R158
Hashimoto K, Matsunaga N, Shimizu M (1994) Effect of vegetable extracts on the transepithelial permeability of the human intestinal Caco-2 cell monolayer. Bioscience. Biotechnol Biochem 58(1994):1345–1346
Jutfelt F (2006) The intestinal epithelium of salmonids: trans-epithelial transport, barrier function and bacterial interactions. PhD Thesis, Department of zoology, zoophysiology, Góteborg University, Sweden
Knudsen D, Uran P, Arnous A, Koppe W, Frøkiær H (2007) Saponin-containing subfractions of soybean molasses induce enteritis in the distal intestine of Atlantic salmon. J Agric Food Chem 55:2261–2267
Knudsen D, Jutfelt F, Sundh H, Sundell K, Koppe W, Frøkiaer H (2008) Dietary soya saponins increase gut permeability and play a key role in the onset of soyabean-induced enteritis in Atlantic salmon (Salmo salar L.). Br J Nutr 100:120–129
Krogdahl A, Bakke-McKellep AM, Baeverfjord G (2003) Effects of graded levels of standard soybean meal on intestinal structure, mucosal enzyme activities and pancreatic response in Atlantic salmon (Salmo salar L.). Aquac Nutr 9:361–371
Marshall WS, Howard JA, Cozzi RRF, Lynch EM (2002) NaCl and fluid secretion by the intestine of the teleost Fundulus heteroclitus: involvement of CFTR. J Exp Biol 205:745–758
Mineo M, Hiroshi H, Norihiro S, Yasuhide O, Fusao T (2002) Melibiose, difructose anhydride III and difructose anhydride IV enhance net calcium absorption in rat small and large intestinal epithelium by increasing the passage of tight junctions in vitro. Nutrient interactions and toxicity. J Nutr 132:3394–3399
Pessi T, Sutas Y, Marttinen A, Isolauri E (1998) Probiotics reinforce mucosal degradation of antigens in rats: implications for therapeutic use of probiotics. J Nutr 128:2313–2318
Poli BM, Parisi G, Zamapacavall G, Iurzan F, Mecatti M, Lupi P, Bonelli A (2003) Preliminary results on quality and quality changes in reared meagre (Argyrosomus regius): body and fillet traits and freshness changes in refrigerated commercial-size fish. Aquac Int 11:301–311
Pratoomyot J, Bendiksen EÅ, Bell JG, Tocher DR (2010) Effects of increasing replacement of dietary fishmeal with plant protein sources on growth performance and body lipid composition of Atlantic salmon (Salmo salar L.). Aquaculture 305:124–132
Robaina L, Izquierdo MS, Moyano FJ, Socorro J, Vergara JM, Montero D, Fernández-Palacios H (1995) Soybean and lupin seed meals as protein sources in diets for gilthead seabream (Sparus aurata): nutritional and histological implications. Aquaculture 130:219–233
Santigosa E, Sánchez J, Médale F, Kaushik S, Pérez-Sánchez J, Gallardo MA (2008) Modifications of digestive enzymes in trout (Oncorhynchus mykiss) and sea bream (Sparus aurata) in response to dietary fish meal replacement by plant protein sources. Aquaculture 282:68–74
Schroede B, Duncker S, Barth S, Bauerfeind R, Gruber AD, Deppenmeier S, Breves G (2006) Preventive effects of the probiotic Escherichia coli strain Nissle 1917 on acute secretory diarrhea in a pig model of intestinal infection. Dig Dis Sci 51(4):724–731
Sundella K, Jutfelta F, Agustsson T, Olsenc RE, Sandbloma E, Hansenc T, Bjornssona BT (2003) Intestinal transport mechanisms and plasma cortisol levels during normal and out-of-season parr–smolt transformation of Atlantic salmon, Salmo salar. Aquaculture 222:265–285
Tacon AGJ, Metian M (2008) Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: trends and future prospects. Aquaculture 285:146–158
Thiel-Demby VE, Humphreys JE, John-Williams LA, Ellens HM, Shah N, Ayrton AD, Polli JW (2009) Biopharmaceutics classification system: validation and learnings of an in vitro permeability assay. Mol Pharm 6:11–18
Torstensen BE, Bell G, Rosenlund GR, Henderson J, Graff IE, Tocher DR, Lie O, Sargent JR (2005) Tailoring of Atlantic salmon (Salmo salar) flesh lipid composition and sensory quality by replacing fish oil with a vegetable oil blend. J Agric Food Chem 53:10166–10178
Torstensen BE, Espe M, Sanden M, Stubhaug I, Waagbo R, Hemre GI, Fontanillas R, Nordgarden U, Hevroy EM, Olsvik P, Berntssen MHG (2008) Novel production of Atlantic salmon (Salmo salar) protein based on combined replacement of fish meal and fish oil with plant meal and vegetable oil blends. Aquaculture 285:193–200
Trischitta F, Faggio C (2006) Effect of the flavonol quercetin on ion transport in the isolated intestine of the eel, Anguilla anguilla. Comp Biochem Physiol C 143:17–22
Trischitta F, Faggio C (2008) Gossypol affects ion transport in the isolated intestine of the seawater adapted eel, Anguilla anguilla. Comp Biochem Physiol A 151:139–143
Acknowledgments
To BIOIBERICA and to SPAROS for providing, respectively, the nucleotides and the diets used in this research. To Fundación Alfonso Martín Escudero for supporting M. A. Saenz de Rodrigáñez, with a postdoctoral fellowship. This research used resources from project PTDC/MAR/104008/2008 (Ministry of Science and Higher Education, Portugal and European Social Funds), PROMAR projects (AQUACOR 31-03-05FEP-003 and NUTRICOR 31-03-05-FEP-19, co-financed by FEDER.) and FCT-“Ciência 2008 program”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Rodrigáñez, M.A.S., Fuentes, J., Moyano, F.J. et al. In vitro evaluation of the effect of a high plant protein diet and nucleotide supplementation on intestinal integrity in meagre (Argyrosomus regius). Fish Physiol Biochem 39, 1365–1370 (2013). https://doi.org/10.1007/s10695-013-9790-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-013-9790-x