Abstract
In the current study, plasma steroid hormones were used to assess the individual variability of Leucoraja erinacea over the course of 12 months, in hopes of further defining its reproductive cycle. No statistical differences in hormone concentrations were observed between the isolated and non-isolated female skates. Monthly E2 concentrations ranged from 1,430 pg ml−1 in August to 3,940 pg ml−1 in March, indicating the presence of mature ovarian follicles and supporting the conclusions from previous studies that L. erinacea is capable of reproducing year-round. Concentrations of E2 were significantly elevated or depressed during some months (February, March, June, July, August, and September) of the year, suggesting that reproductive activity may vary over the annual cycle. Even though monthly P4 concentrations were highly variable, ranging from 82 pg ml−1 in November to 816 pg ml−1 in September, no significant reproductive peaks were observed. In addition, a persistently large variation in E2 and P4 concentrations, indicative of reproductive asynchrony within (mean CV 62 % and CV 69 %, respectively) and between (mean range CV 78 and 125 %, respectively) individual skates, was observed throughout the study. Collectively, the continually high E2 concentrations and variability in both hormones observed in the current study are indicative of an oviparous species that reproduces actively throughout the year. However, the weekly sampling frequency revealed that plasma E2 concentrations, not P4, were more useful to assess reproductive status in asynchronous continuously breeding oviparous elasmobranchs.
Similar content being viewed by others
Introduction
Elasmobranchs (sharks and batoids) constitute a vertebrate group with approximately 1,100 extant species worldwide (Compagno 2001; Hamlett 2005). Global declines in elasmobranch populations, largely due to increased direct and indirect fishing pressures, have raised concerns over their long-term sustainability (Grainger and Garcia 1996; Dulvy et al. 2000, 2003; Awruch et al. 2008). In order to generate reliable population demographic estimates necessary for the successful management of these species, it is essential to understand their life history (Dulvy and Reynolds 2002; Walker 2005; Awruch et al. 2008). A particularly important aspect of life history is reproductive biology (Dulvy and Reynolds 2002; Walker 2005; Awruch et al. 2008). Gathering accurate information, such as the timing of reproduction, quantity of offspring produced, and size and age at maturity, can provide valuable insight into current biomass health as well as the capacity for a species to withstand anthropogenic pressures (Dulvy et al. 2000, 2003; Dulvy and Reynolds 2002; Walker 2005).
The ability to gather accurate reproductive information is often limited by the methods used to collect it. Traditionally, maturity status and reproductive cyclicity of an elasmobranch species have been evaluated by performing gross morphological examinations on euthanized specimens (Castro 1996; Francis et al. 2001; Ruocco et al. 2006; Colonello et al. 2007; Natanson et al. 2007; Oddone et al. 2007; Sulikowski et al. 2007; Whitney and Crow 2007; Cicia et al. 2009; Heupel and Simpfendorfer 2010). Although non-lethal techniques (e.g., intrauterine endoscopy, steroid hormone analysis, ultrasound) have been utilized, lethal sampling still remains the most accurate method to obtain reproductive data to date (Heupel and Simpfendorfer 2010; Hammerschlag and Sulikowski 2011). However, killing specimens to obtain reproductive information has become increasingly problematic, due to the prohibited, threatened, or endangered status of many sharks, skates, and rays (Sulikowski et al. 2007; Awruch et al. 2008). Thus, there is a pressing need to develop new, non-lethal sampling techniques or to refine existing ones, so that reproductive information gathered from them can be regularly incorporated into management plans (Hammerschlag and Sulikowski 2011).
A promising non-lethal approach to obtain reproduction-related information, such as maturity and cyclicity, in elasmobranch species is the determination of plasma steroid hormone concentrations. Although plasma steroid hormones correlate with morphological changes within the reproductive tracts of elasmobranchs (Koob et al. 1986; Tsang and Callard 1987a, b; Rasmussen and Murru 1992; Rasmussen and Gruber 1993; Manire et al. 1995; Snelson et al. 1997; Heupel et al. 1999; Rasmussen et al. 1999; Tricas et al. 2000; Sulikowski et al. 2004, 2005, 2006; Awruch et al. 2008), the ability to establish a link between plasma steroid hormones and the reproductive cycle is more clearly apparent in species that exhibit a defined annual reproductive cycle (Manire et al. 1995; Heupel et al. 1999; Sulikowski et al. 2004). The ability to correlate hormonal changes to the reproductive cycle in species that are reproductively active throughout the year has been hampered by the high degree of individual variability, which is compounded by low sample size (Rasmussen and Gruber 1993; Manire et al. 1995; Rasmussen et al. 1999) and/or reproductive asynchrony (Snelson et al. 1997; Tricas et al. 2000; Kneebone et al. 2007; Sulikowski et al. 2007). Consequently, these studies could only focus on general trends, reducing their ability to discern actual peaks from transient changes in reproductive hormones (Manire et al. 1995; Snelson et al. 1997; Tricas et al. 2000; Kneebone et al. 2007) and perhaps limiting the usefulness of plasma steroid hormone analysis as a stand-alone approach.
The little skate inhabits a broad geographical range extending from Nova Scotia to Cape Hatteras, NC and is considered to be the most common inshore skate species in the Gulf of Maine (GOM; McEachran and Musick 1975). Previous investigations on the little skate have primarily used the frequency of females bearing egg cases and oviposition to establish its reproductive cycle (Bigelow and Schroeder 1953; Richards et al. 1963; Johnson 1979; Palm et al. 2011). While it was reported that the little skate actively reproduces throughout the year, there was disagreement, however, regarding whether this species exhibits one (Bigelow and Schroeder 1953; Palm et al. 2011) or two reproductive peaks (Richards et al. 1963; Johnson 1979).
The ambiguity surrounding the number of reproductive peaks in the little skate may be attributed to the high degree of individual variability throughout the reproductive cycle (Bigelow and Schroeder 1953; Richards et al. 1963; Johnson 1979; Palm et al. 2011). The need to resolve this ambiguity, coupled with the ability to easily maintain this species in a captive environment, make the little skate an ideal candidate for profiling patterns of plasma steroid hormone concentrations over the entire duration of a reproductive cycle in an oviparous elasmobranch. Thus, the goals of the present study were to (1) clarify the ambiguity surrounding the presence and timing of peak(s) during the reproductive cycle of the Gulf of Maine little skate population through analysis of steroid hormones in plasma collected weekly over 1 year; (2) evaluate the variations in weekly plasma steroid hormone concentrations within and between individuals; and (3) establish sampling frequency recommendations based on the degree of individual variability by month, as part of an ideal monitoring program that best reflects the reproductive status of asynchronous continuously breeding elasmobranchs.
Materials and methods
Specimen collection
Female little skates, Leucoraja erinacea, were collected from a 1,600 km2 area centered at 42°54′N and 70°41′W in the Gulf of Maine. Prior to this study (2008–2009), skates were collected by commercial otter trawl, while aboard commercial fishing vessels (n = 5) and by hand (n = 6) during near-shore SCUBA dives throughout 2004–2007. Throughout the study, mortality occurred in the captive population. To offset the loss of those individuals, additional skates were collected during the study by commercial otter trawl in April (n = 4), May (n = 1), and June (n = 1), 2009. All female skates collected for this study were determined to be mature (mean total length 520 mm), based on the Gulf of Maine morphological maturity criteria reported by Cicia et al. (2009).
The captive female little skates were housed at the University of New England’s Marine Science Center in circular tanks (2.0 m × 1.5 m) with an open, flow-through, seawater system with a turnover rate of 38 l min−1. Similar to the wild population, little skates experienced seasonal variations in both water temperature (minimum 5.2 °C in both January and March to maximum temperature of 21.5 °C in August) and photoperiod (ranging from 9:15 light/dark in late December to early January to 15.5:8.5 light/dark in June and early July). For the duration of the study, skates were maintained in three separate tanks, two had male and female skates in equal ratios and one had four female skates isolated from males. The additional skates collected in 2009 were integrated into the two combined gender tanks. Animal husbandry included daily feedings of equal rations of herring and mackerel (Palm et al. 2011). Prior to the beginning of the experiment, each skate was photographed and a Floy spaghetti tag (Floy Tag & Mfg. Inc., Seattle, WA, USA) was inserted at the base of the caudal fin for identification.
Plasma collection
Blood samples were collected for 52 consecutive weeks, beginning in September 2008 and ending in August 2009. Prior to phlebotomy, individual skates were anesthetized using tricaine methanesulfonate (MS-222; Argent Laboratories, Redmond, WA, USA) at a concentration of 0.10 g MS-222 l−1 of seawater (Leonard et al. 1999). Approximately 3 ml of blood was collected from the caudal vein using a double-sided 22 gauge needle attached to a 3 ml lithium heparin vacutainer. After the blood draw and before return to their regular tanks, skates were placed into a recovery tank (130 cm × 100 cm × 10 cm) with flow-through seawater until the effects of MS-222 subsided (i.e., increased mobility and buccal pumping; approximately 5 min). The collected blood samples were then centrifuged at 1,500×g for 5 min, and the plasma was transferred into 1.5-ml eppendorf tubes. Plasma samples were stored at −20 °C until further analysis.
Steroid radioimmunoassay
Plasma concentrations of estradiol (E2) and progesterone (P4) were evaluated to determine seasonal cyclicity and individual variability over an annual reproductive cycle. Hormone concentrations were determined by radioimmunoassays modified from the procedures of Tsang and Callard (1987a), Sulikowski et al. (2004). The radiolabeled steroids used were estradiol [2,4,6,7-³H] (GE Healthcare; http://www.gehealthcare.com/usen-/index.html) and progesterone [1,2,6,7-³H] (Perkin Elmer Life Sciences, Boston, MA, USA). The specifics of the antibodies, titers, and non-radiolabeled steroids were identical to those previously described in Sulikowski et al. (2004).
Briefly, each plasma sample was extracted twice using 10 volumes of diethyl ether. Following each extraction, the aqueous phase was snap frozen in a dry ice and acetone bath. The ether phases were decanted into the same test tube and evaporated under a stream of nitrogen. The dried extracts were then reconstituted in phosphate-buffered saline with 0.1 % gelatin (PBSG) and stored at −20 °C until assay. An aliquot of radiolabeled steroid (1,000 counts min−1) was added to each sample prior to addition of ether for calculating procedural losses. The overall mean recoveries for E2 and P4 were 75 and 76 %, respectively.
Following the separation of free and bound steroid by charcoal adsorption, radioactivity was assessed using a Perkin Elmer Tri-Carb 2900 PR liquid scintillation counter (http://las.perkinelmer.com), and the computer software program AssayZap (Biosoft, http://www.biosoft.com/w/assayzap.htm) was used to calculate the hormone concentrations (pg ml−1) in each plasma sample by interpolations of the standard curves. The intra-assay coefficients of variance were 11.1 % for E2 and 9.4 % for P4, and the inter-assay coefficients of variance were 9.8 % for E2 and 13.3 % for P4.
Statistical analysis
A two-way repeated measures ANOVA was used to evaluate hormone concentrations in female skates between tank treatments (isolated vs combined gender) and by month. Pairwise comparison post-hoc tests were used to assess specific month to month variations in hormone concentrations, and coefficients of variation (CV; standard deviation (SD)/mean) × 100) were used to evaluate the relative variability in hormone values for individuals during each month and over the year. Distributions were determined to be significantly different at α ≤ 0.05 and monthly hormone concentrations are presented as standard error of the mean (SEM).
Results
Plasma steroid hormone concentrations over the reproductive cycle
A total of 485 plasma samples collected from mature female little skates (n = 17) were used to determine circulating concentrations of E2 (n = 263) and P4 (n = 222) over a consecutive 52 week period.
The monthly plasma E2 concentrations in female little skates ranged from 1,430 to 3,940 pg ml−1 and showed considerable variation over the annual cycle (Fig. 1a). When analyzed by two-way repeated measures ANOVA, E2 concentrations significantly differed by month (F df=1, 11 = 2.82, p < 0.01), although there was no significant difference by tank or for the interaction term. Specifically, post-hoc tests revealed that elevated concentrations of E2 occurred in February (which was higher than July, p < 0.01, August, p = 0.01, October, p = 0.03, and December, p = 0.03), March (which was higher than January, p < 0.01, April, p = 0.03, May, p = 0.04, July, p < 0.01, August, p < 0.01, October, p < 0.01, November, p = 0.01, and December, p < 0.01), June (which was higher than July, p = 0.01 and August, p = 0.03) and September (which was higher than July, p = 0.01, August, p = 0.02, October, p = 0.05, and December, p = 0.05), while depressed concentrations occurred in July and August [which were lower than February, March, June, and September; (Fig. 1a)].
Weekly steroid hormone concentrations, averaged by month, over the annual cycle of female L. erinacea related to observed reproduction; a estradiol; b progesterone; and c monthly fecundity defined as the number of egg cases laid per month divided by the number of sexually mature females in a tank (data not collected between February and May 2009). The number of blood samples collected and analyzed for each month is indicated below the curve. Different letters denote significant difference (p < 0.05) between months. The absence of letters or those with the same letter(s) denote no difference (p > 0.05) between month(s)
Over the annual cycle, monthly plasma P4 concentrations ranged from 82 pg ml−1 in November to 816 pg ml−1 in September (Fig. 1b). However, no significant differences by month (F df=1, 11 = 1.67, p = 0.08), by tank, or for the interaction term were revealed following two-way repeated measures ANOVA. Paradoxically, while the high concentration of P4 in September coincided with increased egg case production observed that month, an inverse relationship between P4 and egg case production, however, was observed in August (Fig. 1c).
Variation in steroid hormone concentrations within individual serially sampled skates
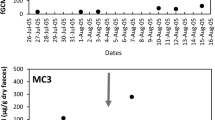
A notable variation in E2 concentrations within individual little skates (mean CV = 62 %) by month was evident over the course of this study ranging from a low CV of 37 % in February to a high CV of 80 % in November. In most cases, months with reduced variation were associated with elevations or depressions in E2 production (i.e., February, March, July, and August with mean CVs 37, 52, 55, and 51 %, respectively), whereas months with higher variation were typically associated with higher concentrations of E2 in the plasma. Although mortality due to periodic sand flea, Talitrus longicornis, infestations impeded our ability to track individual variability over the course of the 52-week study in every skate, the variability represented by the monthly CV values was corroborated visually with the two skates that were able to be tracked for the complete annual cycle (Fig. 2).
The variability of plasma P4 concentrations within individual little skates exhibited a wider range than E2, from a mean CV of 14 % in March to 138 % in October, resulting in an overall mean CV of 74 %. Within each month, P4 concentrations in sampled individuals frequently ranged from non-detectable (below assay sensitivity; ≤12.5 pg ml−1) to concentrations indicative of egg encapsulation (e.g., skate 996 had P4 concentrations that ranged from ≤12.5 to 12,818 pg ml−1 in September). Thus, the proportion of skates collected each month that had undetectable P4 in the plasma notably impacted the degree of individual variability. For example, the months with a larger proportion of samples having below detectable concentrations of P4 were associated with reduced individual variability (i.e., March, April, May, June, and August with mean CV’s of 14, 39, 22, 33, and 50 %, respectively), while the months with a smaller proportion of samples having below detectable concentrations of P4 corresponded with higher individual variability (i.e., July, September, and October, with mean CV’s of 116, 114, and 138 %, respectively).
Variation in steroid hormone concentrations between individual serially sampled skates
There was a high degree of variation in E2 concentrations between individual skates (mean range CV 78 %), with a low CV range of 35 % in February (n = 7) to a high CV range of 103 % in March (n = 8) and June (n = 6; Fig. 3).
Similar to E2, the monthly variability in P4 concentrations between individual little skates encompassed a wide range, from a low CV range of 38 % in July to a high CV range of 187 % in October, with an overall mean CV range of 125 %. The lower variation between individuals (range CV’s below 100 % in March, April, May, July, and August) was generally associated with lower monthly P4 concentrations, while the higher degree of variability between individuals (range CV’s above 100 %, January, February, June, September, October, November, and December) was typically associated with higher monthly P4 concentrations.
Discussion
The persistently high monthly plasma E2 concentrations (1,430–3,940 pg ml−1) found in the present study confirm the year-round recruitment and maintenance of ovarian follicles (Koob et al. 1986). Although the roles of reproductive hormones vary amongst species and reproductive modes, the role of E2, however, remains fairly consistent within the reproductive modes studied to date (i.e., viviparous, histotrophic viviparous, and placental viviparous). In these modes, the primary role of E2 in female elasmobranchs is to regulate egg development during the follicular phase, preparing the reproductive tract for ovulation and subsequent pregnancy (Koob et al. 1986; Tsang and Callard 1987a; Heupel et al. 1999; Rasmussen et al. 1999; Sulikowski et al. 2004, 2005, 2006, 2007; Kneebone et al. 2007). In oviparous species, such as the little skate, the synthesis of vitellogenin (yolk precursor) and subsequent follicular growth have been correlated with increased E2 production (Tsang and Callard 1982; Koob et al. 1986; Perez and Callard 1992; Cicia et al. 2009).
In those species that actively reproduce throughout the year, follicles of varying developmental stages are continually produced within the ovaries (Richards et al. 1963; Koob et al. 1986; Sulikowski et al. 2005; Kneebone et al. 2007). A similar finding of continually high E2 concentrations consistent with ongoing follicle development was observed in the GOM oviparous thorny skates, Amblyraja radiata, and smooth skates, Malacoraja senta, which also exhibit year-round reproductive activity (Kneebone et al. 2007). However, unlike the year-round reproductively active thorny and smooth skates (Kneebone et al. 2007), statistically significant elevations in circulating plasma E2 concentrations were observed in the current study. Here, the highest concentrations of E2 were detected in the late winter and early spring months including a single large peak during March (3,940 pg ml−1, higher than 8 other months) and smaller elevations in February (3,479 pg ml−1, higher than 4 other months), June (3,234 pg ml−1, higher than 2 other months), and September (3,314 pg ml−1, higher than 4 other months). Due to the role of E2 in the little skate (Tsang and Callard 1982; Koob et al. 1986; Perez and Callard 1992; Cicia et al. 2009), significant elevations in E2 concentrations during the aforementioned months indicate that these months may be particularly important for the follicular recruitment necessary to support the increased egg production observed by other studies on the little skate.
In the current study, even though E2 concentrations were robust throughout the year, a direct correlation between E2 and oviposition was not possible due to the four-month gap (February to May) in the sampling of egg cases. However, Palm et al. (2011) reported that viable egg cases are produced throughout the year, with a prolonged and statistically significant peak in egg production during the summer months (June, July, and August). Interestingly, the greatest E2 concentrations in the current study were observed during February (3,479 pg ml−1) and March (3,940 pg ml−1), which are prior to the egg production peak observed by Palm et al. (2011), indicating that this period of time may be particularly important for the follicular recruitment necessary to support the previously observed extended summer peak in oviposition (Fig. 4). Similarly, the July E2 peak reported in the GOM winter skate precedes a period of increased egg production during the fall months (September, October, and November; Sulikowski et al. 2004). Following the E2 peak, reductions in E2 concentrations also correlate with reproductive events such as egg encapsulation, retention, and oviposition (Koob et al. 1986). Accordingly, the low monthly E2 concentrations observed during July (1,553 pg ml−1) and August (1,430 pg ml−1) in the current study provide further evidence of a summer egg production peak in the GOM (Palm et al. 2011). The subsequent smaller elevations in E2 concentrations during June (3,234 pg ml−1) and September (3,314 pg ml−1) would support the declining rate of egg case production during the fall months (Palm et al. 2011).
Peaks in the frequency of egg case deposition from Palm et al. 2011 (dotted square) during the reproductive cycle of L. erinacea compared to monthly estradiol concentrations
In female elasmobranchs studied to date, P4 is generally associated with the maintenance of gestation in both histotrophic aplacental and placental viviparous species (Tsang and Callard 1987a, b; Manire et al. 1995; Koob and Callard 1999) and egg-laying events in oviparous species (Rasmussen et al. 1999; Sulikowski et al. 2004). Previous studies on the little skate indicate that elevations of P4 occur approximately 2 days prior to egg encapsulation for an abbreviated period of time (24–48 h), suggesting that this hormone probably plays a crucial role in ovulation, egg encapsulation, and potentially oviposition (Koob et al. 1986). Overall, a similar pattern was also observed in the present study, where monthly P4 concentrations were relatively low (mean P4 < 600 pg ml−1 during 9 of the months and <700 pg ml−1 during 2 months) with only a single transient increase observed during September (mean P4 > 800 pg ml−1). Further, the P4 concentrations of individual samples in the current study were often below detectable concentrations (<12.5 pg ml−1), consistent with findings in the clearnose skate and draughtboard shark (Rasmussen et al. 1999; Awruch et al. 2008), where P4 concentrations are low except during periods of egg-laying. Even though the overall pattern of P4 concentration is similar, the sampling frequency differs between Koob et al. (1986) and the present study. We noted a single large elevation in September (816 pg ml−1) in conjunction with increased oviposition during that month. However, P4 concentrations (232 pg ml−1; Fig. 1c) did not coincide with the highest observed oviposition (fecundity = 6) during August. This apparent lack of correlation between P4 and egg production in the little skate during August may be misleading, because the weekly sampling regime may have failed to capture the transient periods in which P4 was elevated, whereas Koob et al. (1986) sampled little skates once daily for 20 days during which elevations in P4 concentrations were associated with oviposition. Although P4 likely plays a critical role in reproduction of oviparous species, the inability to observe elevated P4 concentrations in the little skate effectively limits the use of this hormone to ascertain useful, larger-scale information about reproductive status in this species, unless a more frequent sampling regime or a sufficiently larger sample size is used.
Even though valuable information has been obtained through analysis of steroid hormone content in plasma and tissues, the inability to elucidate wide-ranging reproductive trends due to the sampling frequency that is needed (e.g., for P4) in oviparous species and the large variations in hormone concentrations within and between sampled individuals have restricted the use of this non-lethal approach as a primary means to describe reproductive events necessary to manage elasmobranch species (Kneebone et al. 2007; Heupel and Simpfendorfer 2010; Hammerschlag and Sulikowski 2011). To date, correlations between hormone concentrations and morphological parameters are stronger in seasonally breeding elasmobranch species which often exhibit rotating periods of reproductive activity and inactivity (i.e., Rasmussen et al. 1999; Sulikowski et al. 2004, 2005). Conversely, a direct correlation between morphological variables and hormone concentrations (E2) was unable to be detected in the year-round reproductively active smooth skate (Kneebone et al. 2007). Kneebone et al. (2007) speculated that the high degree of variability between individual smooth skates, due to reproductive asynchrony, was responsible for the observed transient elevations in E2 concentrations. Asynchronous reproduction, indeed, may explain why such a high degree of ambiguity continues to exist regarding the presence and timing of reproductive events in year-round, reproductively active species. However, even in elasmobranch species that exhibit synchronous seasonal reproductive cycles, where steroid hormones correlate well with reproductive events (Heupel et al. 1999; Sulikowski et al. 2004, 2007), variability in some species still persists (Snelson et al. 1997; Rasmussen et al. 1999; Tricas et al. 2000). For example, in the seasonally breeding viviparous Atlantic stingray, an increased degree of variability in E2 and P4 was apparent especially during parturition events (E2 2,894.6 ± 805.1 and P4 178.3 ± 17.0). Due to this high variability in seasonal and especially in year-round reproducers, it has become increasingly important to determine the optimal frequency of sample collections, in hopes that the variability will be reduced, thus enabling the use of steroid hormones to more accurately reflect reproductive status and to more efficiently monitor biomass health in the future.
It was, therefore, the purpose of the current study to collect blood samples on a weekly basis from the same female skates to determine whether increasing sampling frequency will provide a clearer relationship between reproductive events and steroid hormone peaks in an asynchronous, continuously breeding oviparous skate species. We found that the variability in monthly plasma E2 concentrations within individual skates was consistently high (mean CV 62 %). Although CVs of steroid hormone concentrations have not been directly reported in previous studies assessing reproductive cyclicity, the large error bars inherent in those data indicate that high variability was also present (i.e., Manire et al. 1995; Snelson et al. 1997; Tricas et al. 2000; Kneebone et al. 2007). The present data also indicate that reductions in variability within individual skates occurred in February, March, July, and August, which most likely were a result of greater synchrony in follicular development just prior to peaks in oviposition (i.e., when there was high mean E2 production in February and March) and during periods of highest oviposition (i.e., which coincided with low mean E2 production in July and August). Interestingly, February was the only month in the current study that displayed low variability within and between individual skates, which coincidentally had high E2 concentrations. This may signify a period of intense folliculogenesis that continues into the summer and early fall. Mature ovarian follicles then stand-ready for egg encapsulation, retention, and oviposition (Koob et al. 1986), supporting a summer egg-laying peak (Palm et al. 2011).
Compared to E2, there was a greater degree of variability in monthly plasma P4 concentrations within (mean CV 74 %) and between (i.e., range CV 0–187 %) individual little skates. This variability is most likely attributed to the relatively narrow window of hormone elevation (24–48 h; Koob et al. 1986) and the reproductive asynchrony in egg-laying between individual female skates. Further, such asynchrony in egg-laying between females (i.e., hours, weeks, or months) and the ensuing transient elevations in P4 concentrations are not uncommon in oviparous species (i.e., Koob et al. 1986; Rasmussen et al. 1999; Awruch et al. 2008). However, some viviparous species also display prolonged elevations in P4 concentrations associated with varying roles in pregnancy (Manire et al. 1995; Koob and Callard 1999). Therefore, while we believe that P4 is probably an unreliable indicator of larger-scale snapshots of reproductive activity in continuously reproducing oviparous skate species, this hormone may be better at assessing the reproductive stock in viviparous species. As a caveat, a more detailed investigation that pinpoints the length of time that plasma E2 and P4 are elevated and the roles they play over the course of pregnancy in viviparous species is needed before any conclusions can be drawn about which hormone is best correlated to particular reproductive events.
In conclusion, the results of the present study suggest that the high plasma E2 concentrations throughout the year in the little skate are consistent with year-round reproduction in oviparous elasmobranch species. Furthermore, the elevations in plasma E2 concentrations during February, March, June, and September lend additional support to the existence of increased reproductive activity observed by previous studies during both the summer and fall months. In addition, despite the reproductive asynchronous nature of little skates, we suggest that the reduced variability associated with significant elevations or depressions in plasma E2 concentrations nonetheless coincided with biologically significant events such as increased follicular recruitment and egg oviposition. On the other hand, because of the narrow window that plasma P4 concentrations have been shown to rise in the little skate, we believe that this limits the ability of this hormone to assess the reproductive cycle or status of continuously breeding female oviparous species unless more frequent (i.e., daily) samples are collected. When daily samples were collected over a discrete period (Koob et al. 1986), plasma P4 provided interesting insights into fundamental reproductive events of the little skates, such as ovulation, egg encapsulation, and oviposition. Overall, the collective life history information obtained so far from studies of elasmobranch species is vital for their management. However, in the current study, sampling frequency was found to be a very important parameter to consider, especially when determining which steroid hormone (i.e., E2, P4) serves as the better indicator of reproductive activity in asynchronous continuously breeding oviparous elasmobranchs like the little skate.
References
Awruch CA, Frusher SD, Pankhurst NW, Stevens JD (2008) Non-lethal assessment of reproductive characteristics for management and conservation of sharks. Mar Ecol Prog Ser 355:277–285. doi:10.3354/meps07227
Bigelow HB, Schroeder WC (1953) Fishes of the Gulf of Maine. Fisheries Bulletin, U.S. Fisheries Wildlife Service, Revision 1.0, 74:577
Castro JI (1996) Biology of the blacktip shark, Carcharhinus limbatus, off the Southeastern United States. Bull Mar Sci 59:508–522
Cicia AM, Driggers WB III, Ingram GW Jr, Kneebone J, Tsang PCW, Koester DM, Sulikowski JA (2009) Size and age estimates at sexual maturity for the little skate Leucoraja erinacea from the western Gulf of Maine, U.S.A. J Fish Biol 75:1648–1666. doi:10.1111/j.1095-8649.2009.02392.x
Colonello JH, Lucifora LO, Massa AM (2007) Reproduction of the angular angel shark (Squatina guggenheim) geographic differences, reproductive cycle, and sexual dimorphism. ICES J Mar Sci 64:131–140. doi:10.1093/icesjms/fsl004
Compagno LJV (2001) Sharks of the world. An annotated and illustrated catalogue of shark species known to date. Vol. 2. Bullhead, mackerel and carpet sharks (Heterodontiformes, Lamniformes and Orectolobiformes). FAO Species Catalogue for Fishery Purposes No. 1, Rome, p 269
Dulvy NK, Reynolds JD (2002) Predicting extinction vulnerability in skates. Conserv Biol 16:440–450. doi:10.1046/j.1523-1739.2002.00416.x
Dulvy NK, Metcalfe JD, Glanville J, Pawson MG, Reynolds JD (2000) Fishery stability, local extinctions, and shifts in community structure in skates. Conserv Biol 14:283–293. doi:10.1046/j.1523-1739.2000.98540.x
Dulvy NK, Sadovy Y, Reynolds JD (2003) Extinction vulnerability in marine populations. Fish Fish 4:25–64. doi:10.1046/j.1467-2979.2003.00105.x
Francis MP, Maolagáin CO, Stevens D (2001) Age, growth, and sexual maturity of two New Zealand endemic skates, Dipturus nasutus and D. innominatus. NZ J Mar Fresh Res 35:831–842. doi:10.1080/00288330.2001.9517045
Grainger RJR, Garcia SM (1996) Chronicles of marine fishery landings (1950–1994): Trend analysis and fisheries potential. FAO Fish Tech T359, p 51
Hamlett WC (2005) Reproductive biology and phylogeny of Chondrichthyes: Sharks, Batoids and Chimeras. Science Publishers, Inc., Enfield, pp 171–299
Hammerschlag N, Sulikowski J (2011) Killing for conservation: the need for alternatives to lethal sampling of apex predatory sharks. Endanger Species Res 14:135–140. doi:10.3354/esr00354
Heupel MR, Simpfendorfer CA (2010) Science or slaughter: need for lethal sampling of sharks. Conserv Biol 24:1212–1218. doi:10.1111/j.1523-1739.2010.01491.x
Heupel MR, Whittier JM, Bennett MB (1999) Plasma steroid hormone profiles and reproductive biology of the epaulette shark, Hemiscyllium ocellatum. J Exp Zool 284:586–594
Johnson GF (1979) The biology of the little skate, Raja erinacea Mitchill 1825, in Block Island Sound, Rhode Island. Master’s thesis. University of Rhode Island, Kingston
Kneebone J, Ferguson DE, Sulikowski JA, Tsang PCW (2007) Endocrinological investigation into the reproductive cycles of two sympatric skate species, Malacoraja senta and Amblyraja radiata, in the western Gulf of Maine. Environ Biol Fishes 80:257–265. doi:10.1007/978-1-4020-9703-4_10
Koob TJ, Callard IP (1999) Reproductive endocrinology of female elasmobranchs: lessons from the little skate (Raja erinacea) and spiny dogfish (Squalus acanthias). J Exp Zool 284:557–574. doi:10.1002/(SICI)1097-010X(19991001)284:5<557:AID-JEZ12>3.0.CO;2-P
Koob TJ, Tsang P, Callard IP (1986) Plasma estradiol, testosterone and progesterone levels during the ovulatory cycle of the skate (Raja erinacea). Biol Reprod 35:267–275. doi:10.1095/biolreprod35.2.267
Leonard JBK, Summers AP, Koob TJ (1999) Metabolic rate of embryonic little skate, Raja erinacea (Chondrichthyes: Batiodea): the cost of active pumping. J Exp Zool 283:13–18
Manire CA, Rasmussen LEL, Hess DL, Hueter RE (1995) Serum steroid hormones and the reproductive cycle of the female bonnethead shark, Sphyrna tiburo. Gen Comp Endocrinol 97:366–376. doi:10.1006/gcen
McEachran JD, Musick JA (1975) Distribution and relative abundance of seven species of skates (Pisces: Rajidae) which occur between Nova Scotia and Cape Hatteras. Fish Bull 73:110–136
Natanson LJ, Sulikowski JA, Kneebone JR, Tsang PC (2007) Age and growth estimates for the smooth skate, Malacoraja senta, in the Gulf of Maine. Environ Biol Fishes 80:293–308. doi:10.1007/s10641-007-9220-y
Oddone MC, Amorim AF, Mancini PL, Norbis W, Velasco G (2007) The reproductive biology and cycle of Rioraja agassizi (Müller and Henle, 1841) (Chondrichthyes: Rajidae) in southeastern Brazil, SW Atlantic Ocean. Scientia Marina 71:593–604. doi:10.3989/scimar.2007.71n3593
Palm BD, Koester DM, Driggers WB, Sulikowski JA (2011) Seasonal variation in fecundity, egg case viability, gestation, and neonate size for little skates, Leucoraja erinacea, in the Gulf of Maine. Environ Biol Fishes 92:585–589. doi:10.1007/s10641-011-9854-7
Perez LE, Callard IP (1992) Identification of vitellogenin in the little skate (Rajah erinacea). Comp Biochem Physiol B 103:699–705. doi:org/10.1016/0305-0491(92)90393-6
Rasmussen LEL, Gruber SH (1993) Serum concentrations of reproductively-related circulating steroid hormones in the free-ranging lemon shark, Negaprion brevirostris. Environ Biol Fishes 38:167–174. doi:10.1007/BF00842913
Rasmussen LEL, Murru FL (1992) Long-term studies of serum concentrations of reproductively related steroid hormones in individual captive carcharhinids. Aust J Mar Freshw Res 43:273–281. doi:10.1071/MF9920273
Rasmussen LEL, Hess DL, Luer CA (1999) Alterations in serum steroid concentrations in the clearnose skate, Raja eglanteria: correlations with season and reproductive status. J Exp Zool 284:575–585. doi:10.1002/(SICI)1097-010X(19991001)284:5<575:AID-JEZ13>3.0.CO;2-I
Richards S, Merriman D, Calhoun LH (1963) Studies on the marine resources of southern New England. IX. The biology of the little skate, Raja erinacea, Mitchell. Bull Bingham Oceanogr Collect 18:5–67
Ruocco NL, Lucifora LO, Díaz de Astarloa JM, Wöhler O (2006) Reproductive biology and abundance of the white-dotted skate, Bathyraja albomaculata, in the Southwest Atlantic. ICES J Mar Sci 63:105–116. doi:10.1016/j.icesjms.2005.08.007
Snelson FF Jr, Rasmussen LEL, Johnson MR, Hess DL (1997) Serum concentrations of steroid hormones during reproduction in the Atlantic stingray, Dasyatis sabina. Gen Comp Endocrinol 108:67–79. doi:10.1006/gcen.1997.6949
Sulikowski JA, Tsang PCW, Howell WH (2004) An annual cycle of steroid hormone concentrations and gonad development in the winter skate, Leucoraja ocellata, from the western Gulf of Maine. Mar Biol 144:845–853. doi:10.1007/s00227-003-1264-8
Sulikowski JA, Tsang PCW, Howell WH (2005) Age and size at sexual maturity for the winter skate, Leucoraja ocellata, in the western Gulf of Maine based on morphological, histological and steroid hormone analyses. Environ Biol Fishes 72:429–441. doi:10.1007/s10641-004-2866-9
Sulikowski JA, Kneebone J, Elzey S, Jurek J, Howell WH, Tsang PCW (2006) Using the composite variables of reproductive morphology, histology and steroid hormones to determine age and size at sexual maturity for the thorny skate Amblyraja radiata in the western Gulf of Maine. J Fish Biol 69:1449–1465. doi:10.1111/j.1095-8649.2006.01207.x
Sulikowski JA, Driggers WB, Ingram GW, Kneebone J, Ferguson DE, Tsang PCW (2007) Profiling plasma steroid hormones: a non-lethal approach for the study of skate reproductive biology and its potential use in conservation management. Environ Biol Fishes 80:285–292. doi:10.1007/s10641-007-9257-y
Tricas TC, Maruska KP, Rasmussen LEL (2000) Annual cycles of steroid hormone production, gonad development, and reproductive behavior in the Atlantic Stingray. Gen Comp Endocrinol 118:209–225. doi:10.1006/gcen.2000.7466
Tsang P, Callard IP (1982) Steroid production by isolated skate ovarian follicular cells. Bull Mt Desert Isl Biol Lab 22:96–97
Tsang PCW, Callard IP (1987a) Morphological and endocrine correlates of the reproductive cycle of the aplacental viviparous dogfish, Squalus acanthias. Gen Comp Endocrinol 66:182–189. doi:10.1016/0016-6480(87)90266-8
Tsang P, Callard IP (1987b) Luteal progesterone production and regulation in the viviparous dogfish, Squalus acanthias. J Exp Zool 241:377–382. doi:10.1002/jez.1402410313
Walker TI (2005) Reproduction in fisheries science. In Reproductive Biology and Phylogeny of Chondrichthyes: Sharks, Batoids and Chimaeras (Hamlett, W. C., volume ed.) in the series: Reproduction Biology and Phylogeny (Jamieson, B. G. M., series ed.) Plymouth: Science Publishers, Inc, pp 81–127
Whitney NM, Crow GL (2007) Reproductive biology of the tiger shark (Galeocerdo cuvier) in Hawaii. Mar Biol 151:63–70. doi:10.1007/s00227-006-0476-0
Acknowledgments
We would like to thank captain J. Jurek of the F.V. “Mystique Lady” and Puggy Jr. of the F.V. “Lady Victoria” for collection of the little skates and the University of New England’s Graduate School and Marine Science Center for funding and use of the wet laboratory facilities. We would also like to extend our gratitude to K. Coutré, R. Knotek, C. Peterson, and A. Traverse-Taylor for assisting with the collection and processing of samples and Dr. David Koester for his thoughtful review of this manuscript. This manuscript represents MSC contribution number 48.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Williams, L.J., Campbell, M.D., Tsang, P.C.W. et al. Using estradiol and progesterone concentrations to assess individual variability in the reproductive cyclicity of captive female little skates, Leucoraja erinacea, from the western Gulf of Maine. Fish Physiol Biochem 39, 1089–1099 (2013). https://doi.org/10.1007/s10695-012-9766-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-012-9766-2