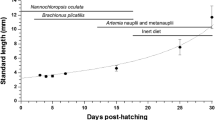
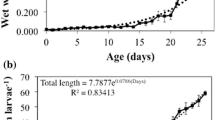
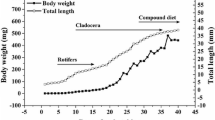
Abstract
Several samples of P. splendida larvae were obtained from eggs until day 60 after hatching (dah) to determine acid and alkaline proteases, trypsin, chymotrypsin, leucine aminopeptidase, α-amylase, lipase, and acid and alkaline phosphatase activities using biochemical techniques. Additionally, SDS–PAGE alkaline protease zymogram and PAGE acid protease zymogram were carried out to identify active isoforms during larviculture. Alkaline protease and chymotrypsin were present at the moment of hatching, increased gradually reaching the maximum values at 35 dah. Trypsin and leucine aminopeptidase activities were low from hatching, increasing gradually as larvae grew. Alkaline protease zymogram showed four zymogens, which appears at different days, remaining present until the end of the larviculture (95.2 kDa at 11 dah, 26.4 kDa at 9 dah, 21.4 kDa at 3 dah, and 23.3 kDa at hatching). Pepsin activity was present at day 7 after hatching and increased progressively until the end of the larviculture. Acid protease zymogram only showed one zymogen (0.65 rf), which appear at 6 dah. Lipase was high at the time of hatching and increased until 15 dah, after which decreased gradually. Amylase was high from the beginning and until 15 dah and then decreased rapidly to almost nothing onward. Alkaline and acid phosphatases presented a high activity at the egg stage, fell slightly during the first feeding and increased again from 20 to 30 dah. Results obtained in this study show that larvae can be fed artificial diets starting on day 10 after hatching.




Similar content being viewed by others
References
Alarcón FJ, Díaz M, Moyano FJ, Abellan E (1998) Characterization and functional properties of digestive proteases in two sparids; gilthead seabream (Sparus aurata) and common dentex (Dentex dentex). Fish Physiol Biochem 19:257–267
Almeida LC, Lundstedt LM, Morales G (2006) Digestive enzyme response of tambaqui (Colossoma macropomum) fed on different levels of protein and lipid. Aquacul Nutr 12:443–450
Álvarez-González CA, Cervantes TM, Tovar RD, Conklin DE, Nolasco H, Gisbert E, Piedrahita R (2006) Development of digestive enzyme in California Halibut (Paralichthys californicus) larvae. Fish Physiol Biochem 31(1):83–93
Álvarez-González CA, Moyano-López FJ, Civera-Cercedo R, Carrasco-Chávez V, Ortiz-Galindo J, Dumas S (2008) Development of digestive enzyme activity in larvae of spotted sand bass (Palabrax maculatofasciatus). I. Biochemical analysis. Fish Physiol Biochem 34:373–384
Álvarez-González CA, Moyano-López FJ, Civera-Cerecedo R, Carrasco-Chávez V, Ortíz-Galindo J, Nolasco-Soria H, Tovar-Ramírez D, Dumas S (2010) Development of digestive enzyme activity in larvae of spotted sand bass Paralabrax maculatofasciatus II: electrophoretic analysis. Fish Physiol Biochem 36:29–37
Anson ML (1938) The estimation of pepsin, tripsin, papain and cathepsin with hemoglobin. J Gen Physiol 22:79–89
Ben NCH, Jian GQ, Martin SK, Wayne GH, Steven MC (2006) Ontogenetic development of digestive enzymes in yellowtail kingfish Seriola lalandi larvae. Aquaculture 260:264–271
Bergmeyer HV (1974) Phosphatases methods of enzymatic analysis, vol 2. Academic Press, New York
Bolasina S, Pérez A, Yamashita Y (2006) Digestive enzymes activity during ontogenetic development and effect of starvation in Japanese flounder, Paralichthys olivaceus. Aquaculture 252:503–515
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brien-MacDonald KO, Joseph AB, Christopher CP (2006) Growth, behavior, and digestive enzyme activity in larval Atlatic cod (Gadus morhua) in relation to rotifer lipid. J Mar Sci 63:275–284
Cahu C, Rønnestad I, Grangier V, Zambonino Infante JL (2004) Expression and activities of pancreatic enzymes in developing sea bass larvae (Dicentrarchus labrax) in relation to intact and hydrolyzed dietary protein; involvement of cholecystokinin. Aquaculture 238:295–308
Cara JB, Moyano FJ, Cardenas S, Fernandez DC, Yufera M (2003) Assessment of digestive enzyme activities during larval development of white bream. J Fish Biol 63:48–58
Caro CC, Mendoza A, Sánchez M (1994) Caracterización del medio ambiente de (Petenia splendida) en lagunas del sur de Quintana Roo. In: Memorias del II Seminario sobre Peces Nativos con uso Potencial en Acuicultura. Del 23 al 26 de mayo. Colegio de Postgraduados, Campus Tabasco. H. Cárdenas, Tabasco, México
Chan R (2004) Efecto de la temperatura sobre el consumo de oxígeno en la mojarra tenguayaca (Petenia splendida) Günter 1862. Undergraduate thesis. Universidad Juárez Autónoma de Tabasco, México
Chávez-Lomelí MO, Mattheeuws AE, Pérez MH (1989) Biología de los Peces del Río San Pedro en vista de determinar su potencial para la piscicultura. INREB-FUCID. Xalapa, Veracruz, México
Chen BN, Qin JG, Kumar MS, Hutchinson W, Clarke S (2006) Ontogenetic development of the digestive system in yellowtail kingfish (Seriola lalandi) larvae. Aquaculture 256:489–500
Chong ASC, Hashim R, Lee LC, Ali AB (2002) Characterization of protease activity in developing discus (Symphysodon aequifasciatus) larva. Aquacult Res 33:663–672
Comabella Y, Mendoza R, Aguilera C, Carrillo O, Hurtado A, García GT (2006) Digestive enzyme activity during early larval development of the Cuban gar (Atractosteus tristoechus) Fish Physiol Biochem 32:147–157
Contreras M (2003) Inversión sexual de las mojarra nativas (Cichlasoma salvini) y (Petenia splendida), mediante administración oral de esteroides sintéticos. Undergraduate thesis. División Académica de Ciencias Biológicas. Universidad Juárez Autónoma de Tabasco, México
DelMar EG, Largeman C, Brodick JW, Geokas MC (1979) A sensitive new substrate for chymotrypsin. Anal Biochem 99:316–320
Díaz-López M, Moyano FJ, García-Carreño LF, Alarcón FJ, Sarasquete MC (1997) Substrate-SDS-PAGE determination of protease activity through larval development in sea bream. Aquacult Inter 5:461–471
Díaz-López M, Moyano FJ, Alarcón FJ, García-Carreño FL, Navarrete del Toro MA (1998) Characterization of fish acid proteases by substrate-gel electrophoresis. Comp Biochem Physiol 121B:369–377
Divakaran S, Kim BG, Ostrowski AC (1999) Digestive enzymes present in Pacific threadfin (Polydactylus sexfilis) (Bloch and Schneider 1801) and Bluefin trevally (Caranx melampygus) (Cuvier 1833). Aquacult Res 30:781–787
Drossou A, Ueberschär B, Rosenthal H, Herzig KH (2006) Ontogenetic development of the proteolytic digestion activities in larvae of (Oreochromis niloticus) fed with different diets. Aquaculture 256:479–488
Erlanger B, Kokowshy N, Cohen W (1961) The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys 95:271–278
Evans RP, Parrish CC, Zhu P, Brown JA, Davis PJ (1998) Changes in phospholipase activity and lipid content during early development of Atlantic halibut (Hippoglossus hippoglossus). Mar Biol 130:369–376
Fernández I, Moyano FJ, Díaz M, Martínez T (2001) Characterization of a-amylase activity in five species of Mediterranean sparid fishes (Sparidae, Teleostei). J Exp Mar Biol Ecol 262:1–12
Furné M, Hidalgo MC, López A, Garcia GM, Morales AE, Domezain A, Domezain EJ, Sanz A (2005) Digestive enzyme activities in Adriatic sturgeon (Acipenser naccarii) and rainbow trout (Oncorhynchus mykiss). A comparative study. Aquaculture 250:391–398
García M (2003) Determinación de la temperatura preferencial y metabolismo de la rutina de la mojarra tenguayaca (Petenia splendida) Günter 1862. Undergraduate thesis. Universidad Juárez Autónoma de Tabasco, México
García-Carreño FL, Dimes LE, Haard NF (1993) Substrate-gel electrophoresis for composition and molecular weight of proteinases or proteinaceous proteinase inhibitors. Anal Biochem 214:65–69
Garcia-Carreño FL, Albuquerque-Cavalcanti C, Navarrete del Toro M, Zaniboni-Filho E (2002) Digestive proteinases of Brycon orbinyanus (Characidae, Teleostei): characteristics and effects of protein quality. Comp Biochem Physiol 132(B):343–352
Guerrero R (2007) Efecto de la temperatura en la proporción de sexos de las mojarras nativas “castarrica” (Cichlasoma urophthalmus) y “tenguayaca” (Petenia splendida). Undergraduate thesis. División Académica de Ciencias Biológicas. Universidad Juárez Autónoma de Tabasco, México
Hamza N, Mhetli M, Kestemont P (2007) Effect of waning age and diets on ontogeny of digestive activities and structures of pikeperch (Sander lucioperca) larvae. Fish Physiol Biochem 33:121–133
Igbokwe EC, Downe AER (1978) Electrophoretic and histochemical comparison of three strains of Aedes aegypti. Comp Biochem Physiol 60B:131–136
Jiménez C (2004) Efecto de la temperatura en el crecimiento de crías de mojarra tenguayaca (Petenia splendida) Günter 1862. Undergraduate thesis. Universidad Juárez Autónoma de Tabasco, México
Jiménez-Martínez LD, Alvarez-González CA, Contreras-Sánchez WM, Márquez-Couturier G, Arias-Rodriguez L, Almeida-Madrigal JA (2009) Evaluation of larval growth and survival in Mexican mojarra, Cichlasoma urophthalmus and bay snook, Petenia splendida under different initial stocking densities. J World Aquacult Soc 40(6):753–761
Jun-Sheng L, Jian-lin L, Ting-ting W (2006) Ontogeny of protease, amylase and lipase in the alimentary tract of hybrid juvenile tilapia (Oreochromis niloticus, Oreochromis aureus). Fish Physiol Biochem 32:295–303
Kim BG, Divakaran S, Brown CL, Ostrowski AC (2001) Comparative digestive enzyme ontogeny in two marine larval fishes: Pacific threadfin (Polydactylus sexfilis) and bluefin trevally (Caranx melampygus). Fish Physiol Biochem 24:225–241
Kumar S, Sharma JG, Chakrabarti R (2000) Quantitative estimation of proteolytic enzyme and structural study of anterior part of intestine of Indian major carp (Catla catla) larvae during ontogenesis. Curr Sci 79:1007– 1101
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lazo JP, Arnold CR (2007) Characterization of digestive enzymes during larval development of red drum (Sciaenops ocellatus). Aquaculture 265:194–205
López LS, Nolasco H, Vega-Villasante F (2003) Characterization of digestive gland esterase - lipase activity of juvenile redclaw crayfish Cherax quadricarinatus. Comp Biochem Physiol 135B:337–347
López-Ramírez G, Cuenca-Soria CA, Alvarez-González CA, Tovar-Ramírez D, Ortiz-Galindo JL, Perales-García N, Márquez-Couturier G, Arias-Rodríguez L, Indy JR, Contreras-Sánchez WM, Gisbert E, Moyano FJ (2010) Development of digestive enzymes in larvae of Mayan cichlid Cichlasoma urophth almus. Fish Physiol Biochem. doi:10.1007/s10695-010-9431-6 (in press)
Ma H, Cahu C, Zambonino J, Yu H, Duan Q, Le Gall M, Mai K (2005) Activities of selected digestive enzymes during larval development of large yellow croaker (Pseudosciaena crocea). Aquaculture 245:239–248
Maraux S, Louvard D, Baratti J (1973) The aminopeptidase from hog-intestinal brush border. Biochem Biophys Acta 321:282–295
Martínez JL (2004) Desarrollo embrionario larval de la mojarra tenguayaca (Petenia splendida) Günter 1862. Undergraduate thesis. División Académica de Ciencias Biológicas, Universidad Juárez Autónoma de Tabasco, México
Moyano FJ, Diaz M, Alarcon FJ, Sarasquete MC (1996) Characterization of digestive enzyme activity during larval development of gilthead seabream (Sparus aurata). Fish Physiol Biochem 15:121–130
Murray HM, Gallant JW, Johnson SC, Douglas SE (2006) Cloning and expression analysis of three digestive enzymes from Atlantic halibut (Hippoglossus hippoglossus) during early development: Predicting gastrointestinal functionality. Aquaculture 252:394–408
Nolting M, Ueberschar B, Rosenthal H (1999) Trypsin activity and physiological aspects in larval rearing of European sea bass (Dicentrarchus labrax) using live prey and compound diets. J Appl Ichthyol 15:138–142
Peña-Martínez R, Dumas S, Villalejo-Fuerte M, Ortíz-Galindo JL (2003) Ontogenetic development of the digestive tract in reared spotted sand bass (Paralabrax maculatofasciatus) larvae. Aquaculture 219:633–644
Rathore RM, Kumar S, Chakrabarti R (2005) Digestive enzyme patterns and evaluation of protease classes in Catla catla (Family: Cyprinidae) during early developmental stages. Comp. Biochem Physiol 142B:98–106
Reséndez A, Salvadores ML (1983) Contribución al conocimiento de la biología del pejelagarto (Lepisosteus tropicus) (Gill) y la tenguayaca (Petenia splendida) (Günter) del Estado de Tabasco. Biótica 8(4):413–426
Ribeiro L, Zambonino-Infante JL, Cahu C, Dinis MT (1999) Development of digestive enzymes in larvae of Solea senegalensis, Kaup 1858. Aquaculture 170:465–473
Ribeiro L, Zambonino-Infante JL, Cahu CL, Dinos MT (2002) Digestive enzymes profile of Solea senegalensis post larvae fed Artemia and a compound diet. Fish Physiol Biochem 27:61–69
Robyt JF, Whelan WJ (1968) The β-amylases. In: Radley JA (ed) Starch and its derivatives. Academic Press, London, pp 477–497
Santiago LMC, Jardon OJ, Jaramillo SG, Reyers AJE, Sanchez VA (1997) Edad, crecimiento y habitos alimenticios de Cichlasoma salvini (Gunther), Cichlasoma urophthalmus (Gunther), Oreochromis niloticus (Linneo) y Petenia splendida (Gunther). Presa Miguel de la Madrid H. “Cerro de Oro” Tuxtepec, Oaxaca. Pp 38. In: V Congreso Nacional de Ictiología. 3 a 5 de Febrero de 1997. Mazatlán, Sinaloa, México. Facultad de Ciencias del Mar. Universidad Autónoma de Sinaloa, Mexico
Souza AAG, Amaral IPG, Albérico RES, Carvalho LB Jr, Bezerra RS (2007) Trypsin-like enzyme from intestine and pyloric caeca of spotted goatfish (Pseudupeneus maculatus). Food Chem 100:1429–1434
Stryer L, Berg JM, Tymoczko JL (2003) Bioquímica: lípidos y membranas celulares. Reverté, quinta edición. España, Barcelona
Suzer C, Saka S, Firat K (2006) Efects of illumination on early life development and digestive enzyme activities in common pandora Pagellus erythrinus L. larvae. Aquaculture 260:86–93
Tengjaroenkul B, Smith BJ, Smith SA, Chatreewongsin U (2002) Ontogenic development of the intestinal enzymes of cultured Nile tilapia, (Oreochromis niloticus L). Aquaculture 211:241–251
Treviño-Carrillo LM, Alvarez-González CA, Perales-García N, Arévalo-Galán L, Uscanga-Martínez A, Márquez-Couturier G, Fernández I, Gisbert E (2010) A histological study of the organogenesis of the digestive system in bay snookPetenia splendida Günther, 1862 from hatching to the juvenile stage. J Appl Ichthyol (in press)
Ueberschär B (1993) Measurement of proteolytic enzyme activity: significance and application in larval fish research. In: Walther BT, Fyhn HJ (eds) Physiological and biochemical aspects of fish development. University of Bergen, Norway
Uscanga A (2006) Determinación de requerimiento de proteína en juveniles masculinizados y no masculinizados de la mojarra tenguayaca (Petenia splendida). Master’s thesis. Universidad Juárez Autónoma de Tabasco, México
Valtierra VMT, Schmitter SJJ (2000) Hábitos alimentarios de las mojarras (Perciformes: Cichlidae) de la laguna Caobas, Quintana Roo, México. Rev Biol Trop 48:2–3
Versaw WK, Cuppett SL, Winters DD, Williams LE (1989) An improved colorimetric assay for bacterial lipase in nonfat dry milk. J Food Sci 54:1557–1558
Vidal-López JM, Alvarez-González CA, Contreras-Sánchez WM, Hernández-Vidal U (2010) Mazculinization of the native cichlid Tenhuayaca, Petenia splendida (Günther, 1862), using Artemia nauplii as vehicle of the steroid 17-α methyltestosterone. Hidrobiológica 19(3):211–216
Walter HE (1984) Proteinases: methods with hemoglobin, casein and azocoll as substrates. In: Bergmeyer HJ (ed) Methods of enzymatic analysis, vol V. Verlag Chemie, Weinham
Williams DE, Reisfeld RA (1964) Disc electrophoresis in polyacrylamide gels: extension to new conditions of pH and buffers. Ann New York Acad Sci 121:373–381
Yanbo W, Zirong X (2006) Effect of probiotics for common carp (Cyprinus carpio) based on growth performance and digestive enzyme activities. Animal Feed Sci Tech 127:283–292
Zambonino-Infante JL, Cahu C (1994) Development and response to a diet change of some digestive enzymes in sea bass (Dicentrarchus labrax) larvae. Fish Physiol Biochem 12:399–408
Zambonino-Infante JL, Cahu CL (2002) Ontogeny of the gastrointestinal tract of marine fish larvae. Comp Biochem Physiol 130C:477–487
Acknowledgments
This study was financed through the research project FOMIX CONACyT-Government of the State of Tabasco “Identificación de ingredientes en alimentos balanceados y su digestibilidad en el cultivo experimental de peces nativos en Tabasco” No. TAB-2005-C06-16260, and the PROMEP project “Ontogenia enzimática y capacidad digestiva de la mojarra tenguayaca Petenia splendida”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uscanga-Martínez, A., Perales-García, N., Álvarez-González, C.A. et al. Changes in digestive enzyme activity during initial ontogeny of bay snook Petenia splendida . Fish Physiol Biochem 37, 667–680 (2011). https://doi.org/10.1007/s10695-011-9467-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-011-9467-2