Abstract
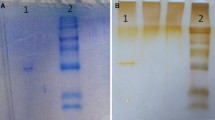
Two forms of rhodanese were purified from the liver of Clarias gariepinus Burchell, designated catfish rhodanese I (cRHD I) and rhodanese II (cRHD II), by ion-exchange chromatography on a CM-Sepharose CL-6B column and gel filtration through a Sephadex G-75 column. The apparent molecular weight obtained for cRHD I and cRHD II was 34,500 ± 707 and 36,800 ± 283 Da, respectively. The subunit molecular weight determined by sodium dodecyl sulphate–polyacrylamide gel electrophoresis was 33,200 ± 283 and 35,100 ± 141 Da for cRHD I and cRHD II, respectively. Atomic absorption spectrophotometric analysis revealed that cRHD II contained a high level of iron (Fe), which presumably was responsible for the brownish colour of the preparation. In contrast, no Fe was identified in cRHD I, and its preparation was colourless. Further characterization of cRHD II gave true Michaelis–Menten constant (Km) values of 25.40 ± 1.70 and 18.60 ± 1.68 mM for KCN and Na2S2O3, respectively, an optimum pH of 6.5 and an optimum temperature of 40°C. The Arrhenius plot of the effects of temperature on the reaction rate consisted of two linear segments with a break occurring at 40°C. The apparent activation energy values from these slopes were 7.3 and 72.9 kcal/mol. Inhibition studies on the cRHD II enzyme showed that the activity of the enzyme was not affected by Mn2+, Co2+, Sn2+, Ni2+ and NH4 +, but Zn2+ inhibited the enzyme considerably.







Similar content being viewed by others
References
Agboola FK, Okonji RE (2004) Presence of rhodanese in the cytosolic fraction of the fruit bat (Eidolon helvum) liver. J Biochem Mol Biol 37(3):275–281
Agency for Toxic Substances and Disease Registry (ATSDR) (1989) Toxicological profile for cyanide. ATSDR/TP-88/12; PB90-162058. Prepared by Syracuse Research Corporation for ATSDR, US Public Health Service, under Contract No. 68-C8- 2004. ATSDR, Atlanta
Aird BA, Heinrikson RL, Westley J (1987) Isolation and characterization of a prokaryotic sulphurtransferase. J Biol Chem 262:17327–17335
Ali A, Al-Qarawi HM, Mousa BH (2001) Tissue and intracellular distribution of rhodanese and mercaptopyruvate sulphurtranferase in ruminants and birds. Vet Res 32:63–70. doi:10.1051/vetres:2001110
Aminlari M, Malekhusseini A, Akrami F, Ebrahimnejad H (2007) Cyanide-metabolizing enzyme rhodanese in human tissues: comparison with domestic animals. Comp Clin Pathol 16(1):47–51. doi:10.1007/s00580-006-0647-x
Anosike EO, Ugochukwu EN (1981) Characterization of rhodanese from cassava leaves and tubers. J Exp Bot 32:1021–1027. doi:10.1093/jxb/32.5.1021
Blumenthal KM, Heinrikson RL (1971) Structural studies of bovine liver rhodanese: I. Isolation and characterization of two active forms of the enzymes. J Biol Chem 246:2430–2437
Bordo D, Bork P (2002) The rhodanese/Cdc25 phosphatase superfamily. Sequence–structure–function relations. EMBO Rep 3:741–746
Bruton MN (1979) The food and feeding behaviour of Clarias gariepinus (Pisces: Clariidae) in Lake Sibaya, South Africa, with emphasis on its role as a predator of cichlids. Trans Zool Soc Lond 35(1):47–114
Chew MY, Boey CG (1972) Rhodanese of tapioca leaf. Phytochemistry 11:167–169. doi:10.1016/S0031-9422(00)89983-5
Clay D (1979) Sexual maturity and fecundity of the African catfish (Clarias gariepinus) with an observation on the spawning behaviour of the Nile catfish (Clarias lazera). Zool J Linn Soc 65:351–365. doi:10.1111/j.1096-3642.1979.tb01100.x
Cleland WW (1970) Steady state kinetics. In: Boyer PB (ed) The enzymes, vol II, 3rd edn. Academic Press, London, pp 1–65
Cosby EQ, Summer JB (1945) Rhodanese. Arch Biochem 7:457–460
Eisler R (1991) Cyanide hazards to fish, wildlife, and invertebrates: a synoptic review. U.S. Fish Wildl Serv Biol Rep 85(1.23):1–55
Environmental Protection Agency (EPA) (1980) Ambient water quality criteria for cyanides. U.S. EPA Rep 440/5-80-037. EPA, Washington D.C.
Ezzi MI, Pascual JA, Gould BJ, Lynch JM (2003) Characterisation of the rhodanese enzyme in Trichoderma spp. Enzyme Microb Technol 32(5):629–634. doi:10.1016/S0141-0229(03)00021-8
Florini JR, Vestling CS (1957) Graphical determination of the dissociation constants for two substrate enzyme systems. Biochim Biophys Acta 25:575–578. doi:10.1016/0006-3002(57)90529-2
Fruton JS, Simmonds S (eds) (1963) Kinetics of enzyme. In: Fruton JS, Simmonds S (eds) General biochemistry, 2nd edn, Wiley, New York, pp 244–283
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum protein by Biuret reaction. J Biol Chem 117:751–766
Himwich WA, Saunders JB (1948) Enzymic conversion of cyanide to thiocyanate. Am J Physiol 53:348–354
Holden AV, Marsden K (1964) Cyanide in salmon and brown trout. Department of Agriculture and Fisheries of Scotland. Freshw Salmon Fish Res Ser 33. Department of Agriculture and Fisheries of Scotland, Edinburgh
Horowitz PM, DeToma F (1970) Improved preparation of bovine liver rhodanese. J Biol Chem 245(6):984–985
Jarabak R, Westley J (1974) Human liver rhodanese: nonlinear kinetic behaviour. Double displacement mechanism. Biochemistry 13(16):3233–3236. doi:10.1021/bi00713a006
Kaur M, Singh K, Rup PJ, Kamboj SS, Saxena AK, Sharma M, Bhagat M, Sood SK, Singh J (2006) A tuber lectin from Arisaema jacquemontii Blume with anti-insect and anti-proliferative properties. J Biochem Mol Biol 39(4):432–440
Keilin D (1929) Cytochrome and respiratory enzymes. Proc R Soc Lond (Biol Sci) 104:206–251. doi:10.1098/rspb.1929.0009
Koj A (1968) Enzymic reduction of thiosulphate in preparations from beef liver. Acta Biochim Pol 15(2):161–169
Koj A, Frendo J, Wojtczak L (1975) Subcellular distribution and intramitochondrial localization of the three sulphurtransferases in rat liver. FEBS Lett 57:42–46
Kuo SM, Lea TC, Stiqanuk MH (1983) Developmental pattern, tissue distribution and subcellular distribution of cysteine: α-ketoglutarateaminotransferase and 3-mercaptopyruvate sulphurtransferases activities in the rat. Biol Neonate 43:23–32
Lameed GA, Obadara PG (2006) Eco-development impact of coca-cola industry on biodiversity resources at Asejire area, Ibadan; Nigeria. J Fish Int 1(4):55–62
Leduc G (1978) Deleterious effects of cyanide on early life stages of Atlantic salmon (Salmo salar). J Fish Res Board Can 35:166–174
Leduc G, Pierce RC, McCracken IR (1982) The effects of cyanides on aquatic organisms with emphasis upon freshwater fishes. National Research Council of Canada (NRCC) Publ 19246. NRCC/CNRC, Ottawa
Lee CH, Hwang JH, Lee YS, Cho KS (1995) Purification and characterization of mouse liver rhodanese. J Biochem Mol Biol 28:170–176
Lieske CN, Clark CR, Zoeffel LD (1996) Temperature effects in cyanolysis using elemental sulphur. J Appl Toxicol 16:171–175. doi:10.1002/(SICI)1099-1263(199603)16:2<171::AID-JAT327>3.0.CO;2-R
Micha JC (1973) Etude des populations piscicoles de l'Ubangui et tentative de selection et d'adaptation de quelques especes a l'etang de pisciculture. Centre Technique Forestier Tropical, Nogent-sur-Marne
Montgomery RD (1965) The medical significance of cyanogens in plant foodstuffs. Am J Clin Nutr 17:103–113
Nagahara N, Nishino T (1996) Role of amino acid residues in the active site of rat liver mercaptopyruvate sulphurtransferases. J Biol Chem 271:27395–27401. doi:10.1074/jbc.271.44.27395
Nagahara N, Okazaki T, Nishino T (1995) Cytosolic mercaptopyruvate sulphurtransferase is evolutionarily related to mitochondrial rhodanese. Striking similarity in active site, amino acid sequence and the increase in mercaptopyruvate sulphurtransferase activity of rhodanese by site directed mutagenesis. J Biol Chem 270:16230–16235. doi:10.1074/jbc.270.27.16230
Nagahara N, Ito T, Minam M (1999) Mercaptopyruvate sulphurtransferase as a defence against cyanide toxications; molecular properties and mode of detoxification. Histol Histopathol 14:1277–1286
Ogata K, Xing D, Volini M (1989) Bovine mitochondrial rhodanese is a phosphoprotein. J Biol Chem 246(5):2718–2725
Ploegman JH, Drent G, Kalk KH, Hol WG (1978) Structure of bovine liver rhodanese. I. Structure determination at 2.5 Å resolution and a comparison of the conformation and sequence of its two domains. J Mol Biol 123:557–594. doi:10.1016/0022-2836(78)90207-3
Russell J, Weng L, Kein PS, Heinrikson RL (1978) The covalent structure of bovine liver rhodanese. J Biol Chem 253:8102–8108
Schlesinger P, Westley J (1974) An expanded mechanism for rhodanese catalysis. J Biol Chem 249:780–788
Segel IH (ed) (1975) Enzymes. In: Segel IH (ed) Biochemical calculations; 2nd edn. Wiley, New York, pp 278–281
Smith LL, Broderius SJ, Oseid DM, Kimball GL, Koenst WM (1978) Acute toxicity of hydrogen cyanide to freshwater fishes. Arch Environ Contam Toxicol 7:325–337
Smith LL, Broderius SJ Jr, Oseid DM, Kimball GL, Koenst WM, Lind DT (1979) Acute and chronic toxicity of HCN to fish and invertebrates. U.S. Environ. Prot. Agency Rep. 600/3-79-009, 129 pp
Smith J, Urbanska KM (1986) Rhodanese activity in Lotus corniculatus sensu-lato. J Nat Hist 20(6):1467–1476. doi:10.1080/00222938600770991
Sorbo BH (1951) On the properties of rhodanese. Acta Chem Scand 5:724–726. doi:10.3891/acta.chem.scand.05-0724
Sorbo BH (1953a) Crystalline rhodanese. Enzyme catalyzed reaction. Acta Chem Scand 7:1137–1145. doi:10.3891/acta.chem.scand.07-1137
Sorbo BH (1953b) Crystalline rhodanese. Acta Chem Scand 7:1129–1136. doi:10.3891/acta.chem.scand.07-1129
Sorbo BH (1955) Rhodanese. In: Sidney PL, Kaplan NO (eds) Methods of enzymology, vol 2. Academic Press, New York, pp 334–337
Sorbo BH (1957) Enzyme transfer of sulphur from mercaptopyruvate to sulphate or sulphonates. Biochim Biophys Acta 24:324–329. doi:10.1016/0006-3002(57)90201-9
Taniguichi T, Kimura T (1974) Role of 3-mercaptopyruvate sulphurtransferase in the formation of the iron chromophore of adrenal ferredoxin. Biochim Biophys Acta 364:284–295
Tolba MK (1982) Development without destruction. Evolving environmental perceptions. Dublin, Tycooly. Nat Resour Environ Ser 12:197
Towill LE, Drury JS, Whitfield BL, Lewis EB, Galyan EL, Hammons AS (1978) Reviews of the environmental effects of pollutants vs. cyanide. U.S. Environmental Protection Agency (EPA) Rep 600/1-78-027. EPA, Washington D.C.
Ulmer DD, Vallee BL (1972) Role of metals in sulphurtranferases activity. Annu Rev Biochem 32:86–90
Vazquez E, Gazzaniga S, Polo C, Batlle A (1997) Mitochondrial and cytosolic rhodanese from liver of DAB-treated mice. III. Inhibition kinetic studies. Cancer Biochem Biophys 15(4):285–293
Villarejo M, Westley J (1963) Mechanism of rhodanese catalysis of thiosulphate oxidation-relation. J Biol Chem 238:4016–4060
Volini M, DeToma F, Westley J (1967) Dimeric structure and zinc content of bovine liver rhodanese. J Biol Chem 242:5220–5225
Wang SF, Volini M (1968) Studies on the active site of rhodanese. J Biol Chem 243:5465–5470
Warburg O (1911) Inhibition of the action of prussic acid in living cells. Hoppe Seylers Z Physiol Chem 76:331–346
Westley J (ed) (1980) Rhodanese and the sulphane pool. In: Jakoby WB (ed) Enzymatic basis of detoxification, vol 2. Academic Press, New York, pp 245–259
Whitaker JR (1972) Effect of temperature on enzyme catalysed reaction. In: Whitaker JR (ed) Principles of enzymology for the food science. Marcel Dekker, New York, pp 319–348
Wokes F, Willimott SG (1951) The determination of cyanide in seed. J Pharm Pharmacol 3:905–916
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akinsiku, O.T., Agboola, F.K., Kuku, A. et al. Physicochemical and kinetic characteristics of rhodanese from the liver of African catfish Clarias gariepinus Burchell in Asejire lake. Fish Physiol Biochem 36, 573–586 (2010). https://doi.org/10.1007/s10695-009-9328-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-009-9328-4