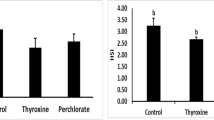
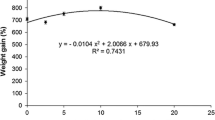
Abstract
Effects of oral administration of l-thyroxine (T4) on growth performance, body composition, and some aspects of nutritional physiology were investigated in two slow-growing air-breathing fish (Channa punctatus and Heteropneustes fossilis) under laboratory conditions (LD 12:12 at 25°C). The results indicate that irrespective of the species, fish fed diets containing lower doses of T4 (up to 50 mg kg−1 of diet in C. punctatus and up to 100 mg kg−1 of diet in H. fossilis) showed significantly (P < 0.05) higher growth (in terms of live weight and length gain, specific growth rate, percentage gain in body weight and condition factor), low feed conversion ratio, high nutrient retention, high apparent protein digestibility, and high digestive enzyme activity. Viscero-somatic (VSI) and hepato-somatic (HSI) values were also high in fish fed on low dietary T4 levels. Liver glycogen levels decreased with the increase in the dietary T4 levels, indicating its utilization during growth. Muscle glycogen levels in H. fossilis coincided with high growth at 100 mg of T4. Observation of the postprandial excretion of metabolites (N–NH4 + and o-PO4 −) indicated significantly (P < 0.05) low levels in aquaria water where the fish were fed diets with low T4 levels. These studies further revealed that feeding fishes on higher T4 levels (above 50 mg in C. punctatus and above 100 mg in H. fossilis) not only repressed growth performance and nutrient retention, but also affected carcass composition by lowering protein accumulation (muscle and carcass protein) and energy assimilation. These studies revealed a biphasic action of thyroxine, i.e., the hormone at lower doses is anabolic, while at higher doses it acts as a catabolic agent, indicating that feeding fishes on higher doses can be detrimental to their growth and metabolism. In summary, the results of the present study show that feeding H. fossilis and C. punctatus on low doses of T4 enhances growth, decreases excretion of metabolites, and increases nitrogen retention. These observations suggest that T4 supplementation of the diet may have practical utility in the culture of slow-growing fish species.
Similar content being viewed by others
References
Ansal MD, Kaur K (1998) Relative efficacy of dietary administration of 3,5,3′-triiodothyronine (T3) to different stages of an Indian major carp, Cirrhinus mrigala (Hamilton): growth and economics. Aquac Res 29:835–841
AOAC (Association of Official Analytical Chemists) (2000) Official methods of analysis. Assoc. Off. Anal. Chem. Inc. Arlinglon, USA, p 684
APHA (American Public Health Association), (1998) Standard methods for the examination of water and waste water. APHA, AWWA, WPFC, 16ED, New York
Arul V (1987) Effect of l-thyroxine on growth and food utilization in Channa striatus. In: The first indian fisheries forum (Abstracts), Asian Fisheries Society, Mangalore, p 59
Brett JR, Groves TDD (1979) Physiological energetics. In: Hoar WS, Randall DJ, Brett JR (eds) Fish physiology. vol VIII. Academic Press, New York, pp 279–352
Cho CY, Slinger SJ, Bayley HS (1982) Bioenergetics of salmonid fishes: energy intake, expenditure and productivity. Comp Biochem Physiol 73(B):25–41
Degani G, Gallagher ML (1986) The influence of 3, 5, 3′-triiodo-l-thyronine on growth, survival and body composition of slow growing development elvers (Anguilla rostrata L.). Comp Biochem Physiol 84:7–11
Donaldson EM, Fagerlund UHM, Higgs DA, McBride JR (1979) Hormonal enhancement of growth. In: Hoar WS, Randall DJ, Brett JR (eds) Fish physiology, vol VIII. Academic Press, New York, pp 455–597
Dubois M, Gilles KA, Hamilton JK, Robers PA, Smith F (1956) Colorimetric method for determination of sugars and related compounds. Anal Chem 28:350–356
Eales JG (1979) Thyroid functions in cyclostomes and fishes. In: Barrington EJW (ed) Hormones and evolution, vol I. Academic Press, New York, pp 341–436
Fagerlund UHM, McCallum L, Higgs DA, McBride RJ, Plotnikoff MD, Dosanjh BS (1984) Diet composition as a factor in the anabolic efficacy of 3,5,3′-triiodo-l-thyronine administered orally to steelhead trout (Salmo gairdneri). Aquaculture 36:49–59
Farbridge KR, Leatherland JF (1988) Interaction between ovine growth hormone and triiodo-l-thyronine on metabolic reserve of rainbow trout, Salmo gairdneri. Fish Physiol Biochem 5:141–151
Fontaine M, Baraduc M, Hatey J (1953) Influence de la thyroxinisation sur la teneur en glycogene due foie des poissons teleosteens. CR Soc Biol 147:214–216
Furukawa A, Tuskahara H (1966) On the acid digestion methods for determination of chromic oxide as an indicator substance in the study of digestibility in fish. Bull Jpn Soc Sci Fish 32:502–506
Gross WL, Fromm PO, Roelofs EW (1963) Relationship between thyroid and growth in green sunfish. Lepomis cyanellus (Rafinesque). Trans Am Fish Soc 92:401–408
Henken AM, Lucas H, Tijssen PAT, Machiels MAM (1986) A comparison between methods used to determine the energy content of feed, fish and faeces samples. Aquaculture 58:195–201
Higgs DA (1974) Influence of nutritional state on thyroid hormone metabolism in the brook trout, Salvelinus fontinalis (Mitchill). Ph.D. Thesis, Univ. of Manitoba, Winnipeg
Higgs DA, Donaldson EM, Dye HM, McBride JR (1976) Influence of bovine growth hormone and l-thyroxine on growth, the muscle composition and histological structure of the gonads, thyroid, pancreas and pituitary of coho salmon (Oncorhynchus kisutch). J Fish Res Board Can 33:1585–1603
Higgs D, Fagerlund U, McBride JR, Dye H, Donaldson E (1977) Influence of combinations of bovine growth hormone, 17α-methyltestosterone and l-thyroxine on growth of yearling coho salmon (Oncorhynchus kisutch). Can J Zool 55:1048–1056
Higgs DA, Fagerlund UHM, McBride JR, Eales JG (1979) Influence of orally administered l-thyroxine or 3,5,3′-triiodo-l-thyronine on growth, food consumption and food conversion in underyearling coho salmon (Oncorhynchus kisutch). Can J Zool 25:1974–1979
Higgs DA, Fagerlund UHM, Eales JG, McBride JR (1982) Application of thyroid and steroid hormones as anabolic agents in fish culture. Comp Biochem Physiol 73B:143–176
Higgs DA, Dosanjh BS, Uin LM, Himick BA, Eales JG (1992) Effect of dietary lipid and carbohydrate levels and chronic 3,5,3′-triiodo-l- thyronine treatment on growth, appetite, food and protein utilization and body composition of immature rainbow trout Oncorhynchus mykiss at low temperature. Aquaculture 105:175–190
Kumar A, Swarup N, Singh DP (1991) Effect of thyroxine administration on growth and morphological parameters of larvae of Cyprinus carpio (Linn.). J Agril Sci Res 33:86–92
Lam TJ (1973) Experimental induction of changes in fishes. J Singapore Natl Acad Sci 3:188–191
Lam TJ (1980) Thyroxine enhances larval development and survival in Sarotherodon (Tilapia) mossambicus. Aquaculture 21:287–291
Lam TJ (1985) Role of thyroid hormone on larval growth and development in fish. In: Lofts B, Holmes WN (eds) Current trends in comparative endocrinology. Hong Kong University Press, Hong Kong, pp 481–485
Lam TJ, Sharma R (1985) Effects of salinity and thyroxine on larval survival, growth and development in the carp Cyprinus carpio. Aquaculture 44:201–212
Leray C, Bonnet B, Febvre A, Vallet F, Pic P (1970) Quelques activities peripheriques des hormones thyroidiennes observees chez Mugil auratus L. (Teleosteen Mugilide). Ann d’ Endocrinol Paris 31:567–572
Lin SJ, Tin YY, Chang CF (1994) The stimulatory effects of 3,5,3′-triiodo-l-thyronine on the content of liver soluble protein in tilapia. J Fish Soc Taiwan 21:361–368
Malhotra S, Garg SK (2003) Effect of recombinant bovine growth hormone (rbGH) administration on growth, body composition and gut proteolytic enzymes activity in fingerlings of Channa punctatus (Bloch). J Aquac 11:49–58
Malhotra, S Garg SK (2004) Effect of immersion treatment in bovine insulin on growth, nutrient retention and proteolytic enzyme activity in Channa punctatus (Bloch). J Aquac 12:35–42
Matty A, Lone K (1985) Hormonal control of protein deposition. In: Cowey CB, Mackie AM, Bell JG (eds) Nutrition and feed in fish. Academic Press, London, pp 147–167
McBride JR, Higgs DA, Fagerlund UHM, Buckley JT (1982) Thyroid and steroid hormones: potential for control of growth and smoltification of salmonids. Aquaculture 28:201–209
Medda AK, Ray AK (1979) Effect of thyroxine and analogs on protein and nuceleic acid contents of liver and muscle of lata fish (Ophiocephalus punctatus). Gen Comp Endocrinol 37:74–80
Middleton WRJ (1971) Thyroid hormones and the gut. Gut 12:172–177
Moon HYL, MacKenzie DS, Gatlin DMIII (1994) Effects of dietary thyroid hormones on the red drum (Sciaenops ocellatus). Fish Physiol Biochem 12:369–380
Narayansingh T, Eales JG (1975) Effect of thyroid hormones on in vivo [I14C] l-leucine incorporation in plasma and tissue protein of brook trout (Salvelinus fontinalis) and rainbow trout (Salmo gairdneri). Comp Biochem Physiol 52B: 399–405
Piferrer F (2001) Endocrine sex control strategies for the feminization of teleost fish. Aquaculture 197:229–281
Piferrer F, Donaldson EM (1994) Uptake and Clearance of exogenous estradiol -17β and testosterone during the early development of coho salmon (Oncorhynchus kisuech), including eggs, alevins and fry. Fish Physiol Biochem 13:219–232
Reddy PK, Lam TJ (1992) Effect of thyroid hormones on morphogenesis and growth of larvae and fry of telescopic-eye black goldfish. Carassius auratus. Aquaculture 107:383–394
Sawhney SK, Singh R (2000) Introductory practical biochemistry. Narosa Publishing House, India
Smith MAK, Thorpe A (1977) Endocrine effects on nitrogen excretion in the euryhaline teleost, Salmo gairdneri. Gen Comp Endocrinol 32:400–406
Steffens W (1989) Principles of fish nutrition. Horwood, New York
Sumagaysay-Chavoso NS (2003) Nitrogen and phosphorus digestibility and excretion of different-sized groups of milkfish (Chanos chanos Forsskal) fed formulated and natural food-based diets. Aquac Res 34:407–418
Walter HE (1984) Method of enzymatic analysis. Verlag Chemie, Weinheim
Weatherley AH, Gill HS (1987) Influence of hormones. In: Weatherley AH, Gill HS (eds) The biology of fish growth. Academic Press, New York, pp 177–206
Woo NYS, Chung AS, Ng TB (1991) Influence of oral administration of 3,5,3′-triiodo-l-thyronine on growth, digestion, food conversion and metabolism in the underyearling red sea bream—Chrysophrys major. J Fish Biol 34:459–468
Acknowledgements
The research work of the author was supported through a research grant from C(b) Zoo-11-ICAR-NATP (World Bank).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garg, S.K. Effect of oral administration of l-thyroxine (T4) on growth performance, digestibility, and nutrient retention in Channa punctatus (Bloch) and Heteropneustes fossilis (Bloch). Fish Physiol Biochem 33, 347–358 (2007). https://doi.org/10.1007/s10695-007-9166-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-007-9166-1