Abstract
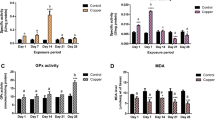
The biochemical status of antioxidant defences of larvae from the commercial, benthic fish, Solea senegalensis, were studied over a period of 28 days from hatching. The parameters studied were: catalase (EC 1.11.1.6), total glutation peroxidase activity (sum of the selenium dependent and independent forms) (EC 1.6.4.2), DT-diaphorase (EC 1.6.99.2), and as an indication of conjugative detoxifying metabolism, the enzyme glutathione S-transferase (GST; EC 2.5.1.18). Oxidative damage was evaluated by the formation of malondialdehyde (MDA) as an indication of lipid peroxidation (LP). Metallothionein (MT) levels were measured by differential pulse polarography and stress proteins (HSP 70 and HSP 60) were detected by immunoblotting, using commercial antibodies. The presence of catalytic activities was observed from hatching day and tended to increase during larval development. Significant changes were seen in most enzymes at day 3, when the larvae finished their endogenous feeding and started to feed on rotifers. DT-diaphorase activities were similar in both NADH and NADPH-dependent forms and, in turn, were about 10-times lower than their correspondent reductases. LP sharply increased at 19 and 28 days post-hatch (dph), suggesting a saturation of the antioxidant defences during metamorphosis. MT showed high values during the endogenous phase and the lowest value corresponded to day 3, when the egg-yolksac was fully reabsorbed; a steady-state value was reached thereafter. Stress proteins were present at all stages, showing distinctiveness in the molecular weight of HSP60 at hatching day and 1 dph. Quantitatively, both forms were significantly elevated at 3 dph, with their ratio (HSP70/HSP60) decreasing over time.
Similar content being viewed by others
Abbreviations
- CAT:
-
catalase
- GST:
-
glutathion S-trasferase
- dph:
-
days post hatch
- DT-D:
-
DT-diaphorase (NAD(P)H quinone oxidoreductase)
- DCPIP:
-
dichlorophenol indophenol
- HSP:
-
heat shock protein
- LP:
-
lipid peroxidation
- MDA:
-
malondialdehide
- MT:
-
metallothionein
- PUFA:
-
polyunsaturated fatty acids
- ROS:
-
reactive oxygen species
- t-GPX:
-
total glutathion peroxidase
References
A. Aceto F. Amicarelli P. Sacchetta B. Dragan T. Bucciarelli L. Masciocco M. Miranda C. Di Ilio (1994) ArticleTitleDevelopmental aspects of detoxifying enzymes in fish (Salmo iridaeus) Free Radical Res. 21 285–294 Occurrence Handle1:CAS:528:DyaK2cXmvFOntbk%3D
A.E. BasuN. Todgham P.A. Ackerman P.M. BibeauM.R.NakanoK. Schulte Iwama G.K. (2002) ArticleTitleHeat shock protein genes and their functional significance in fish Gene 295 173–183 Occurrence Handle10.1016/S0378-1119(02)00687-X Occurrence Handle12354651
J.W. Bauman J. Liu Y.P. Liu C.D. Klaassen (1991) ArticleTitleIncrease in metallothionein produced by chemicals that induce oxidative stress Toxicol. Appl. Pharmacol. 110 347–354 Occurrence Handle1:CAS:528:DyaK3MXlslOjs70%3D Occurrence Handle1891778
E. Cadenas (1995) ArticleTitleAntioxidant and prooxidant functions of DT-diaphorase in quinone metabolism Biochem. Pharmacol. 49 127–140 Occurrence Handle10.1016/S0006-2952(94)00333-5 Occurrence Handle1:CAS:528:DyaK2MXjsVKhs78%3D Occurrence Handle7530954
E.E. Deane N.Y.S. Woo (2003) ArticleTitleOntogeny of thyroid hormones, cortisol, hsp70 and hsp90 during silver sea bream larval development Life Sci. 72 805–818 Occurrence Handle10.1016/S0024-3205(02)02334-2 Occurrence Handle1:CAS:528:DC%2BD38XptlGisbo%3D Occurrence Handle12479979
P. Prisco ParticleDe R. Scudiero V. Carginale A. Capasso E. Parisi B. Petrocellis ParticleDe (1991) ArticleTitleDevelopment changes of metallothionein content and synthesis in sea urchin, Paracentrotus lividus embryos Cell. Inter. Rep. 15 305–317 Occurrence Handle10.1016/0309-1651(91)90169-J
M.T. Dinis L. Ribeiro F. Soares C. Sarasquete (1999) ArticleTitleA review on the cultivation potential of Solea senegalensis in Spain and Portugal Aquaculture 176 27–38 Occurrence Handle10.1016/S0044-8486(99)00047-2
H. Esterbauer R.J. Schaur H. Zollner (1991) ArticleTitleChemistry and biochemistry of 4-hydroxynonenal malonaldehyde and related aldehydes Free Radic. Biol. Med. 11 81–128 Occurrence Handle10.1016/0891-5849(91)90192-6 Occurrence Handle1:CAS:528:DyaK3MXmsFSnsbo%3D Occurrence Handle1937131
M.E. Feder G.E. Hoffman (1999) ArticleTitleHeat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology Annu. Rev. Physiol. 61 243–282 Occurrence Handle10.1146/annurev.physiol.61.1.243 Occurrence Handle1:CAS:528:DyaK1MXitVejs7o%3D Occurrence Handle10099689
C. Fernández-Díaz M. Yúfera J.P. Cañavate F.J. Moyano F.J. Alarcón M. Díaz (2001) ArticleTitleGrowth and physiological changes during metamorphosis of Senegal sole reared in the laboratory J. Fish Biol. 58 1086–1097
P.J. Gavaia M.T. Dinis M.L. Cancela (2002) ArticleTitleOsteological development and abnormalities of the vertebral column and caudal skeleton in larval and juvenile stages of hatchery-reared Senegal sole (Solea senegalensis) Aquaculture 211 305–323 Occurrence Handle10.1016/S0044-8486(02)00167-9
S.G. George (1994) Enzymology and molecular biology of phase II xenobiotic-conjugating enzymes in fish D.C. Malins G.K. Ostrander (Eds) Aquatic Toxicology, Molecular, Biochemical and Cellular Prespectives CRC Press Boca Raton 37–85
B. Halliwell J.M.C. Gutteridge (2000) Free Radicals in Biology and Medicine EditionNumber3 Oxford University Press Oxford 936
G.K. Iwama P.T. Thomas R.B. Forsyth M.M. Vijayan (1998) ArticleTitleHeat shock protein expression in fish Rev. Fish Biol. Fish. 8 35–56 Occurrence Handle10.1023/A:1008812500650
P.H. Krone Z. Lele J.B. Sass (1997) ArticleTitleHeat shock genes and the heat shock response in zebrafish embryos Biochem. Cell. Biol. 75 487–497 Occurrence Handle10.1139/bcb-75-5-487 Occurrence Handle1:CAS:528:DyaK1cXitFCitbc%3D Occurrence Handle9551174
U.K. Laemmli (1970) ArticleTitleCleavage of structural proteins during the assembly of the head of bacteriophage T4 Nature 22 680–685
S. Lewis R.D. Handy B. Cordi Z. Billinghurst M.H. Depledge (1999) ArticleTitleStress proteins (HSPs): methods of detection and their use as an environmental biomarker Ecotoxicology 8 351–368 Occurrence Handle10.1023/A:1008982421299 Occurrence Handle1:CAS:528:DC%2BD3cXis1yru7w%3D
D.R. Livingstone L. Förlin S. George (1994) Molecular biomarkers and toxic consequences of impact by organic pollution in aquatic organisms D.W. Sutcliffe (Eds) Water Quality and Stress Indicators in Marine and Freshwater Systems: Linking Levels of Organisation Freshwateer Biological Association Ambleside, U.K 154–171
D.R. Livingstone (2001) ArticleTitleContaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms Mar. Pollut. Bull. 42 656–666 Occurrence Handle10.1016/S0025-326X(01)00060-1 Occurrence Handle1:CAS:528:DC%2BD3MXlslGhsbk%3D Occurrence Handle11525283
O.H. Lowry N.J. Rosebrough A.L. Farr R.J. Randall (1951) ArticleTitleProtein measurement with Folin phenol reagent J.␣Biol. Chem. 193 265–275 Occurrence Handle1:CAS:528:DyaG38XhsVyrsw%3D%3D Occurrence Handle14907713
T. Luckenbach H. Ferling M. Gernhöfer H-R. Köhler R-D. Negele E. Pfefferle R. Triebskorn (2003) ArticleTitleDevelopmental and subcellular effects of chronic exposure to sub-lethal concentrations of ammonia, PAH and PCP mixtures in brown trout (Salmo trutta f. fario L.) early life stages Aquat. Toxicol. 65 39–54 Occurrence Handle10.1016/S0166-445X(03)00107-3 Occurrence Handle1:CAS:528:DC%2BD3sXmsVKit78%3D Occurrence Handle12932700
G. Mourente R. Vázquez (1996) ArticleTitleChanges in the content of total lipid, lipid classes and their fatty acids of developing eggs and unfed larvae of the Senegal sole, Solea senegalensis Kaup Fish Physiol. Biochem. 15 221–235 Occurrence Handle1:CAS:528:DyaK28XkvVKrs74%3D
G. Mourente D.R. Tocher E. Diaz-Salvago A. Grau E. Pastor (1999) ArticleTitleStudy of the n-3 highly unsaturated fatty acids requirement and antioxidant status of Dentex dentex larvae at the Artemia feeding stage Aquaculture 179 291–307 Occurrence Handle10.1016/S0044-8486(99)00166-0 Occurrence Handle1:CAS:528:DyaK1MXksFans7Y%3D
G. Mourente D.R. Tocher E. Diaz A. Grau E. Pastor (1999) ArticleTitleRelationships between antioxidants, antioxidant enzyme activities and lipid peroxidation products during early development in Dentex dentex eggs and larvae Aquaculture 179 309–324 Occurrence Handle10.1016/S0044-8486(99)00167-2 Occurrence Handle1:CAS:528:DyaK1MXksFans7c%3D
Olafson R.W., Olsson P.E. (1991). Electrochemical detection of metallothionein. In: Metallobiochemistry. Part B. Metallothionein and Related Molecules. Edited by J.F. Riordan, and B.L. Valle, Meth. Enzymol. 205: 205–213
G. Parra I. Rønnestad M. Yufera (1999) ArticleTitleEnergy metabolism in eggs and larvae of the Senegal sole J. Fish Biol. 55 205–214 Occurrence Handle1:CAS:528:DyaK1MXotVGgsL0%3D Occurrence Handle10.1111/j.1095-8649.1999.tb01056.x
L.D. Peters D.R. Livingstone (1996) ArticleTitleAntioxidant enzyme activities in embryologic and early larval stages of turbot J. Fish Biol. 49 986–997 Occurrence Handle1:CAS:528:DyaK2sXjs1Knuw%3D%3D
L.D. Peters C. Porte D.R. Livingstone (1994) ArticleTitle7-ethoxyresorufin O-deethylase (EROD) and antioxidant enzyme activities in larvae of sardine (Sardina pilchardus) from the North coast of Spain Mar. Pollut. Bull. 28 299–304 Occurrence Handle10.1016/0025-326X(94)90154-6 Occurrence Handle1:CAS:528:DyaK2cXltFOjs7g%3D
LD. Peters S.S. O’Hara DR. Livingstone (1996) ArticleTitleBenzo(a)pyerene metabolism and xenobiotic-stimulated reactive oxygen species generation by subcellular fractions of larvae of turbot (Scophthalmus maximus L.). Comp Biochem. Physiol. 114 221–227
L.D. Peters C. Porte D.R. Livingstone (2001) ArticleTitleVariation of antioxidant enzyme activities of sprat (Sprattus sprattus) larvae and organic contaminant levels in mixed Zooplankton from the Southern North Sea Mar. Pollut. Bull. 42 1087–1095 Occurrence Handle10.1016/S0025-326X(01)00088-1 Occurrence Handle1:CAS:528:DC%2BD3MXos1Whtrg%3D Occurrence Handle11763220
N.A. Porter S.E. Caldwell K.A. Mills (1995) ArticleTitleMechanisms of free radical oxidation of unsaturated lipids Lipids 30 277–290 Occurrence Handle1:CAS:528:DyaK2MXltFCqtLc%3D Occurrence Handle7609594
I.I. Rudneva (1999) ArticleTitleAntioxidant system of Black Sea animals in early development Comp. Biochem. Physiol. 122 265–271 Occurrence Handle1:STN:280:DyaK1M3gs1SmsA%3D%3D
R. Rueda-Jasso L.E.C. Conceiçäo J.W. Dias E. De Coen E. Gomes J.F. Rees F. Soares M.T. Dinis P. Sorgeloos (2004) ArticleTitleEffect of dietary non-protein energy levels on condition and oxidative status of Senegalese sole (Solea senegalensis) juveniles Aquaculture 231 417–433 Occurrence Handle10.1016/S0044-8486(03)00537-4 Occurrence Handle1:CAS:528:DC%2BD2cXpsVOiuw%3D%3D
T. Sakai H. Murata M. Endo T. Shimomura K. Yamauchi T. Tto T. Yamaguchi H. Nakajima M. Fukudome (1998) ArticleTitleSevere oxidative stress is thought to be a principal cause of jaundice of yellowtail Seriola quinqueradiata Aquaculture 160 205–214 Occurrence Handle10.1016/S0044-8486(97)00304-9 Occurrence Handle1:CAS:528:DyaK1cXitVKktbo%3D
.M. Sanders (1993) ArticleTitleStress proteins in aquatic organisms: an environmental perspective Crit. Rev. Toxicol. 23 49–75 Occurrence Handle1:CAS:528:DyaK3sXkt1Crs7Y%3D Occurrence Handle8471160
M. Solé C. Porte D. Barceló (2000) ArticleTitleVitellogenin induction and other biochemical responses in carp, Cyprinus carpio, after experimental injection with 17α-ethynylestradiol Arch. Environ. Contam. Toxicol. 38 494–500 Occurrence Handle10.1007/s002449910065 Occurrence Handle10787101
Stoscheck C.M. (1990). Quantitation of protein. In: Guide to Protein Purification. Edited by P.D. Murray. Meth. Enzymol. 182: 50–67
R. Oost Particlevan der J. Beyer N.P.E. Vermeulen (2003) ArticleTitleFish bioaccumulation and biomarkers in environmental risk assessment: a review Environ. Toxicol. Pharmacol. 13 57–149 Occurrence Handle10.1016/S1382-6689(02)00126-6
R. Vázquez S. González A. Rodríguez G. Mourente (1994) ArticleTitleBiochemical composition and fatty acid content of fertilized eggs, yolksac stage larvae and first feeding larvae of the Senegal sole (Solea senegalensis Kaup) Aquaculture 119 273–286 Occurrence Handle10.1016/0044-8486(94)90182-1
A. Viarengo B. Burlando M. Cavaletto B. Marchi E. Ponzano J. Blasco (1999) ArticleTitleRole of metallothionein against oxidative stress in the mussel Mytilus galloprovincialis Am. J. Physiol. 277 (Regulatory Integrative Comp. Physiol) 46 1612–1619
H. Westernhagen ParticleVon (1988) Sublethal effects of pollutants on fish eggs and larvae W.S. Hoas D.J. Randall (Eds) Fish Physiology Academic Press San Diego 253–346
C.E. Wheelock T.A. Baumgartner J.W. Newman M.F. Wolfe R.S. Tjeerdema (2002) ArticleTitleEffect of nutritional state on Hsp60 levels in the rotifer Brachionus plicatilis following toxicant exposure Aquat. Toxicol. 61 89–93 Occurrence Handle10.1016/S0166-445X(02)00044-9 Occurrence Handle1:CAS:528:DC%2BD38Xnt1Gjsbc%3D Occurrence Handle12297373
F.-L. Yeh T. Hsu (2000) ArticleTitleDetection of a spontaneous high expression of heat shock protein 70 in developing zebrafish (Danio rerio) Biosci. Biotechnol. Biochem. 64 592–595 Occurrence Handle10.1271/bbb.64.592 Occurrence Handle1:CAS:528:DC%2BD3cXit1Gntrk%3D Occurrence Handle10803959
M. Yúfera G. Parra R. Santiago M. Carrascosa (1999) ArticleTitleGrowth, carbon, nitrogen and caloric content of Solea senegalensis (Pises: Soleidae) from egg fertilisation to metamorphosis Mar. Biol. 134 43–49 Occurrence Handle10.1007/s002270050523
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Solé, M., Potrykus, J., Fernández-Díaz, C. et al. Variations on stress defences and metallothionein levels in the Senegal sole, Solea senegalensis, during early larval stages. Fish Physiol Biochem 30, 57–66 (2004). https://doi.org/10.1007/s10695-004-6786-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-004-6786-6