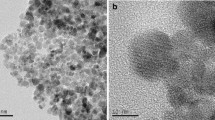
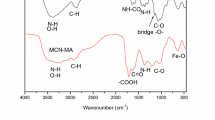
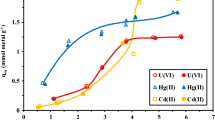
The efficacy of using chitosan cryogel modified with pyridoxal-5´-phosphate for extraction of 152,154Eu(III) from aqueous solutions was established. Enhancement of the sorption properties of CCTZ-PP in comparison to CCTZ is due to the possibility of formation of a bond between Eu(III) with ionized phosphate groups. CCTZ-PP can be recommended for separation and concentraton of 152,154Eu(III) from low-salt technogenic solutions and natural media.



Similar content being viewed by others
References
A. J. Varma, S. V. Deshpande, and J. F. Kennedy, Carbohydr. Polym., 55, 77–93 (2004).
R. Drot and E. Simoni, Langmuir, 15, 4820–4827 (1999).
M. G. Almazan-Torres, N. Finck, et al., in 11 th Intern. Conf. on the Chemistry and Migration Behavior of Actinides and Fission Products in the Geosphere “MIGRATION ‘07”, Munich, Germany (2007), p. 163.
K. A. Bol’shakov (ed.), Chemistry and Technology of Rare and Stray Elements [in Russian], Part 2, Vysshaya Shkola, Moscow (1976).
V. I. Spintsin and L. I. Martynenko, Coordination Chemistry of Rare-Earth Elements [in Russian], MGU, Moscow (1976).
M. T. Valentini Ganzerli, Caramella V. Crespi, and L. Maggi, J. Radioanal. Nucl. Chem., 254, NO. 1, 3–7 (2002).
F. Macašek, J. Radioanal. Nucl. Chem., 240, NO. 1, 251–259 (2002).
P. Benez, K. Stamberg, et al., J. Radioanal. Nucl. Chem., 254, NO. 2, 231–239 (2002).
Y. Takahashi, Y. Minai, et al., J. Radioanal. Nucl. Chem., 234, No. 1–2, 277–282 (1998).
T. Ozaki, T. Kimura, et al., Chem. Lett., 32, No. 7, 560–561 (2003).
W. S. Wan Ngah, C. S. Endud, and R. Mayanar, React. Funct. Polym., 50, NO. 2, 181–190 (2002).
B. N. Narasinga Rao, C. Ramakrishnan, and P. Balaram, J. Biosci., 1, NO. 1, 35–47 (1979).
V. V. Nikonorov, R. V. Ivanov, et al., Vysokomol. Soedin., A52, NO. 8, 1433–1446 (2010).
N. R. Kil’deeva, V. V. Nikonorov, et al., in: Vestn. Mosk. Gos. Tekstil. Univ., A. N. Kosygin MGTU, Moscow (2009), pp. 59–63.
F. Albert Cotton and Geoffrey Wilkinson, Advanced Inorganic Chemistry, 5th ed., Wiley, NewYork (1988).
R. Griesbach, Theory and Practice of Ion Exchange, Academic, Berlin (1957).
A. G. Kasatkin, Basic Processes and Equipment in Chemical Engineering [in Russian], Khimiya, Moscow (1973).
Yu. A. Kokotov and V. A. Pasechnik, Equilibrium and Kinetics of Ion Exchange [in Russian], Khimiya, Leningrad (1970).
The Research was Conducted with the Financial Support of the Federal Target Program “Scientific and Scientific Pedagogical Personnel of Innovative Russia” in 2009–2013. State Contract No. 16.740.11.0059.
Author information
Authors and Affiliations
Additional information
Translated from Khimicheskie Volokna, No. 6, pp. 22–26, November-December, 2010
Rights and permissions
About this article
Cite this article
Veleshko, I.E., Nikonorov, V.V., Veleshko, A.N. et al. Sorption of Eu(III) from solutions of covalently cross-linked chitosan cryogels. Fibre Chem 42, 364–369 (2011). https://doi.org/10.1007/s10692-011-9287-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10692-011-9287-2