Abstract
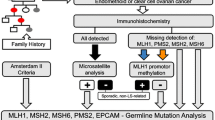
Lynch syndrome is an autosomal dominant cancer predisposition syndrome caused by germline mutations in one of the mismatch repair (MMR) genes: MLH1, MSH2, MSH6 and PMS2. Clinically, Lynch syndrome is characterized by early onset (45 years) of colorectal cancer (CRC), as well as extra-colonic cancer. Male and female carriers of Lynch syndrome-associated mutations have different lifetime risks for CRC and in women endometrial cancer (EC) may be the most common tumor. Whenever Amsterdam criteria are not fulfilled, the currently recommended laboratory screening strategies involve microsatellite instability testing and immunohistochemistry staining of the tumor for the major MMR proteins. The aim of this study was to estimate the frequency of MMR deficiencies in women diagnosed with EC who are at-risk for Lynch syndrome. Thirty women diagnosed with EC under the age of 50 years and/or women with EC and a first degree relative diagnosed with a Lynch syndrome-associated tumor were included. To assess MMR deficiencies four methods were used: multiplex PCR, Single Strand Conformation Polymorphism, Immunohistochemistry and Methylation Specific–Multiplex Ligation-dependent Probe Amplification. Twelve (40%) patients with EC fulfilling one of the inclusion criteria had results indicative of MMR deficiency. The identification of 5 women with clear evidence of MMR deficiency and absence of either Amsterdam or Bethesda criteria among 10 diagnosed with EC under the age of 50 years reinforces previous suggestions by some authors that these women should be considered at risk and always screened for Lynch syndrome after informed consent.

Similar content being viewed by others
Abbreviations
- CRC:
-
Colorectal cancer
- DAB:
-
Diaminobenzidine
- DNA:
-
deoxyribonucleotide acid
- EC:
-
Endometrial cancer
- FH:
-
Familial history
- FIPe—HCPA:
-
Fundação de Incentivo à Pesquisa do Hospital de Clínicas de Porto Alegre
- IHC:
-
Immunohistochemistry
- MLH1:
-
MutL homolog 1
- MMR:
-
Mismatch repair
- MSH2:
-
MutS homolog 2
- MSH6:
-
MutS homolog 6
- MS:
-
Microsatellite stability
- MSI:
-
Microsatellite instability
- MS–MLPA:
-
Methylation specific multiplex ligation-dependent probe amplification
- PCR:
-
Polymerase chain reaction
- PMS2:
-
Postmeiotic segregation increased 2
- SSCP:
-
Single strand conformational polymorphism
- Tris–EDTA:
-
Tris base, ethylenediaminetetracetic acid, disodium salt
- UFRGS:
-
Universidade Federal do Rio Grande do Sul
References
Hadley DW, Jenkins JF, Steinberg SM et al (2008) Perceptions of cancer risks and predictors of colon and endometrial cancer screening in women undergoing genetic testing for Lynch syndrome. J Clin Oncol 26(6):948–954
Buttin BM, Powell MA, Mutch DG et al (2004) Increased risk for hereditary nonpolyposis colorectal cancer-associated synchronous and metachronous malignancies in patients with microsatellite instability-positive endometrial carcinoma lacking MLH1 promoter methylation. Clin Cancer Res 10(2):481–490
Black D, Soslow RA, Levine DA et al (2006) Clinicopathologic significance of defective DNA mismatch repair in endometrial carcinoma. J Clin Oncol 24(11):1745–1753
de la Chapelle A (2004) Genetic predisposition to colorectal cancer. Nat Rev Cancer 4(10):769–780
Millar AL, Pal T, Madlensky L et al (1999) Mismatch repair gene defects contribute to the genetic basis of double primary cancers of the colorectum and endometrium. Hum Mol Genet 8(5):823–829
Vasen HF (2005) Clinical description of the Lynch syndrome [hereditary nonpolyposis colorectal cancer (HNPCC)]. Fam Cancer 4(3):219–225
Arnold CN, Goel A, Blum HE et al (2005) Molecular pathogenesis of colorectal cancer: implications for molecular diagnosis. Cancer 104(10):2035–2047
Lu HK, Broaddus RR (2005) Gynecologic cancers in Lynch syndrome/HNPCC. Fam Cancer 4(3):249–254
Modica I, Soslow RA, Black D et al (2007) Utility of immunohistochemistry in predicting microsatellite instability in endometrial carcinoma. Am J Surg Pathol 31(5):744–751
Lancaster JM, Powell CB, Kauff ND et al (2007) Society of gynecologic oncologists education committee statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol 107(2):159–162
Hewitt MJ, Wood N, Quinton ND et al (2006) The detection of microsatellite instability in blind endometrial samples—a potential novel screening tool for endometrial cancer in women from hereditary nonpolyposis colorectal cancer families? Int J Gynecol Cancer 16(3):1393–1400
Lagerstedt Robinson K, Liu T, Vandrovcova J et al (2007) Lynch syndrome (hereditary nonpolyposis colorectal cancer) diagnostics. J Natl Cancer Inst 99(4):291–299
Meyer LA, Broaddus RR, Lu KH (2009) Endometrial cancer and Lynch syndrome: clinical and pathologic considerations. Cancer Control 16(1):14–22
Broaddus RR, Lynch HT, Chen LM et al (2006) Pathologic features of endometrial carcinoma associated with HNPCC: a comparison with sporadic endometrial carcinoma. Cancer 106(1):87–94
Matthews KS, Estes JM, Conner MG et al (2008) Lynch syndrome in women less than 50 years of age with endometrial cancer. Obstet Gynecol 111(5):1161–1166
Peltomäki P (2005) Lynch syndrome genes. Fam Cancer 4(3):227–232
Kariola R, Raevaara TE, Lönnqvist KE et al (2002) Functional analysis of MSH6 mutations linked to kindreds with putative hereditary non-polyposis colorectal cancer syndrome. Hum Mol Genet 11(11):1303–1310
Abdel-Rahman WM, Peltomäki P (2008) Lynch syndrome and related familial colorectal cancers. Crit Rev Oncog 14(1):1–22 (discussion 23–31)
Watson P, Riley B (2005) The tumor spectrum in the Lynch syndrome. Fam Cancer 4(3):245–248
Oliveira Ferreira F, Napoli Ferreira CC, Rossi BM et al (2004) Frequency of extra-colonic tumors in hereditary nonpolyposis colorectal cancer (HNPCC) and familial colorectal cancer (FCC) Brazilian families: an analysis by a Brazilian hereditary colorectal cancer institutional registry. Fam Cancer 3(1):41–47
Geary J, Sasieni P, Houlston R et al (2008) Gene-related cancer spectrum in families with hereditary non-polyposis colorectal cancer (HNPCC). Fam Cancer 7(2):163–172
Berends MJ, Wu Y, Sijmons RH et al (2003) Toward new strategies to select young endometrial cancer patients for mismatch repair gene mutation analysis. J Clin Oncol 21(23):4364–4370
Zhang L (2008) Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part II. The utility of microsatellite instability testing. J Mol Diagn 10(4):301–307
Iacopetta B, Hamelin R (1998) Rapid and nonisotopic SSCP-based analysis of the BAT-26 mononucleotide repeat for identification of the replication error phenotype in human cancers. Hum Mutat 12:355–360
The National Comprehensive Cancer Network, Fort Washington, PA. http://www.nccn.org. Cited 6 Mar 2009
Bonis PA, Trikalinos TA, Chung M, Chew P, Ip S, DeVine DA, Lau J (2007) Hereditary nonpolyposis colorectal cancer: diagnostic strategies and their implications. Evid Rep Technol Assess (Full Rep) (150):1–180
Shia J, Klimstra DS, Nafa K et al (2005) Value of immunohistochemical detection of DNA mismatch repair proteins in predicting germline mutation in hereditary colorectal neoplasms. Am J Surg Pathol 29(1):96–104
Shia J (2008) Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn 10(4):293–300
Halvarsson B, Lindblom A, Rambech E et al (2004) Microsatellite instability analysis and/or immunostaining for the diagnosis of hereditary nonpolyposis colorectal cancer? Virchows Arch 444(2):135–141
MRC-Holland, Amsterdam, The Netherlands. http://www.mrc-holland.com. Cited 16 Feb 2009
Jeuken JW, Cornelissen SJ, Vriezen M et al (2007) MS–MLPA: an attractive alternative laboratory assay for robust, reliable, and semiquantitative detection of MGMT promoter hypermethylation in gliomas. Lab Invest 87(10):1055–1065
Acknowledgments
We are indebted to the patients and their family members who agreed to participate in this study and to Fundação de Incentivo à Pesquisa do Hospital de Clínicas de Porto Alegre (FIPe-HCPA) and Programa de Pós-Graduação em Ciências Gastroenterológicas (UFRGS) who provided funding for this study. We also thank Drs. Miguel Varela and Gabriel Prolla for patient referrals. We are also indebted to Jan Schouten (MRC-Holland, Amsterdam, The Netherlands) for the technical support with MS–MLPA. We thank Dr Vinicius Duval da Silva and Thiago Giugliani (Laboratório de Patología, Hospital São Lucas, Porto Alegre, Brazil) for their stimulating discussions and technical support in IHC protocols, and Aishameraine Venes Schmidt (UFRGS) for help with the data analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cossio, S.L., Koehler-Santos, P., Pessini, S.A. et al. Clinical and histomolecular endometrial tumor characterization of patients at-risk for Lynch syndrome in South of Brazil. Familial Cancer 9, 131–139 (2010). https://doi.org/10.1007/s10689-009-9297-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-009-9297-x