Abstract
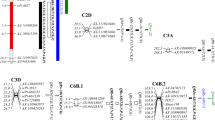
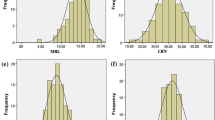
Root system traits have positive effects on wheat grain yield, particularly in drought environments. Root traits are difficult to manipulate using conventional selection procedures. Marker-assisted-selection (MAS) could be helpful for the improvement of root morphological traits. A recombinant inbred line (RIL) population of 168 lines derived from the cross Iran #49 × Yecora Rojo was used to map quantitative trait loci (QTLs) for root traits at mid-tillering stage for one season and for root and shoot traits at plant maturity for two seasons using two different subsets. The RILs were grown in sand-tube experiments in a glasshouse under well-watered conditions. Longest root (LR), total root length longer 30 cm (TRL30), shallow root weight (roots between 0 and 30 cm, SRW), deep root weight (roots bellow 30 cm, DRW), total root biomass (RBio), ratio of root to shoot (RTS) and to plant (RTP) biomass were measured at mid-tillering. At maturity, number of days to booting (DTB), to heading (DTH), to anthesis (DTA), and to maturity (DTM), plant height (PH), flag leaf area (FLA), number of tillers (NTP) and spikes (NSP) per plant, number of grains (NGP), grain weight (GW), grain yield (GY) per plant, LR, SRW, DRW, RBio, PBio, and RTP were measured. At mid-tillering, a total of 18 putative QTLs were detected with individual QTL accounted for between 6.5 and 26.5 % of the variation in the traits. The QTLs were distributed on chromosomes 1B, 2A, 2D, 4B, 6B, 7A, and 7D. A major and two minor QTLs were identified for LR, with the major QTL (qLR-2D) explaining 26.5 % of variation. Two QTLs were detected for DRW on chromosome 4B between markers Gwm6 and Sukkula.1220 that together explained 23.1 % of variation. One region between marker Wmc198 and Cfa2263 on chromosome 2A contained four QTLs affecting PH, SRW, RTS, and RTP. At maturity, 70 putative QTLs were detected across the two seasons with a single QTL accounted for between 7.7 and 40.6 % of variation in the traits. Three major colocalized QTLs for SRW, DRW, and RBio were identified on chromosome 2D between markers Wms515 and Wms102 that accounted for 19.8, 20.5, and 22.4 % 0f variation, respectively. Two major colocalized QTLs for SRW and RBio were detected on chromosome 3A that explained 17.8 and 13.4 % of variation, respectively. One major QTL for DRW was identified on chromosome 1B that accounted for 20.3 % of variation. Chromosome 2B harbored major QTLs for GY, NGS, and NGP. A major QTL cluster was detected on chromosome 2D and on chromosome 4A relating 11 and eight QTLs for phenological periods, root traits, RTS, and RTP, indicating pleiotropic effects on these traits. Of the four common root traits studied at mid-tillering and at maturity, only SRW had linked QTLs on chromosome 2A at both stages of plant growth, indicating selection for root traits at seedling stage alone may not be effective in changing root morphological characteristics at later stages of plant growth. It appeared that chromosome 2A, 2D, and 4B harbored genes regulating growth of root traits at early and later stages of plant growth. The molecular markers closely linked to QTLs for root and shoot traits may be used in wheat breeding program using MAS procedures.








Similar content being viewed by others
References
Bai C, Liang Y, Hawkesford MJ (2013) Identification of QTLs associated with seedling root traits and their correlation with plant height in wheat. J Exp Bot 64:1745–1753
Börner A, Röder M, Korzun A (1997) Comparative molecular mapping of GA insensitive Rht loci on chromosome 4B and 4D of common wheat (Triticum aestivum L.). Theor Appl Genet 95:1133–1137
Christopher J, Christopher M, Jennings R, Jones S, Fletcher S, Borrell A, Manschadi AM, Jordan D, Mace E, Hammer G (2013) QTL for root angle and number in a population developed from bread wheats (Triticum aestivum L.) with contrasting adaptation to water-limited environments. Theor Appl Genet 126:1563–1574
Churchill GA, Doerge RW (1994) Empirical threshold values for quantitative trait mapping. Genetics 138:963–971
Dunbabin V, Diggle A, Rengel Z, Gill G, Mendham N (2003) Breeding more productive grain crops—could selecting the right rooting traits help? In: Solutions for a better environment, Proceedings of the 11th Australian agronomy conference, Geelong Vic, Australian Society of Agronomy, Published on CDROM. ISBN 0-9750313-0-9. Accessed 2–6 Feb 2003
Ehdaie B (1995) Variation in water-use efficiency and its components in wheat: II. Pot and field experiments. Crop Sci 35:1617–1626
Ehdai B, Waines JG (1997) Chromosomal location of genes influencing plant characters and evapotranspiration efficiency in bread wheat. Euphytica 96:363–375
Ehdaie B, Waines JG (2006) Determination of a chromosome segment influencing rooting ability in wheat-rye 1BS-1RS recombinant lines. J Genet Breed 60:71–76
Ehdaie B, Whitkus RW, Waines JG (2003) Root biomass, water-use efficiency, and performance of wheat–rye translocations of chromosomes 1 and 2 in spring bread wheat ‘Pavon’. Crop Sci 43:710–717
Ehdaie B, Merhaut DJ, Ahmadian S, Hoops AC, Khuong T, Layne AP, Waines JG (2010) Root system size influences water-nutrient uptake and nitrate leaching potential in wheat. J Agron Crop Sci 196:455–466
Ehdaie B, Layne AP, Waines JG (2012) Root system plasticity to drought influences grain yield in bread wheat. Euphytica 186:219–232
Ehdaie B, Maheepala DC, Bektaş H, Waines JG (2014) Phenotyping and genetic analysis of root and shoot traits of recombinant inbred lines of bread wheat under well-watered conditions. J Crop Improv 28:834–851
Ellis MH, Spielmeyer W, Gale KR, Rebetzke GJ, Richards RA (2002) ‘Perfect’ markers for the Rht B1b and Rht D1b dwarfing genes in wheat. Theor Appl Genet 105:1038–1042
Fu X, Qi Z, Li S (2011) QTL detecion for water-soluble oligosaccharide content of grain in common wheat. Mol Plant Breed 2:68–74
Gregory PJ, Bengough AG, Grinev D, Schmidt S, Thomas WTB, Wojciechowski T, Young IM (2009) Root phenomics of crops: opportunities and challenges. Funct Plant Biol 36:922–929
Gupta PK, Balyan HS, Edwards KJ, Isaac P, Korzun V, Roder M, Gautier MF, Joudrier P, Schlatter AR, Dubcovsky J, De la Pena RC, Khairallah M, Penner G, Hayden MJ, Sharp P, Keller B, Wang RCC, Hardouin JP, Jack P, Leroy P (2002) Genetic mapping of 66 new microsatellite (SSR) loci in bread wheat. Theor Appl Genet 105:413–422
Guyomarc’h H, Sourdille P, Charmet G, Edwards KJ, Bernard M (2002) Characterization of polymorphic microsatellite markers from Aegilops tauschii and transferability to the D genome of bread wheat. Theor Appl Genet 104:1164–1172
Hurd EA (1968) Growth of roots of seven varieties of spring wheat at high and low moisture levels. Agron J 60:201–205
Hurd EA (1974) Phenotype and drought tolerance in wheat. Agric Meteorol 14:39–55
Kabir MR, Liu G, Guan P, Wang F, Khan AA, Ni Z, Yao Y, Hu Z, Xin M, Peng H, Sun Q (2015) Mapping QTLs associated with root traits using two different populations in wheat (Triticum aestivum L.). Euphytica 206(1):175–190. doi:10.1007/s10681-015-1495-z
Kadam S, Singh K, Shukla S, Goel S, Vikram P, Pawar V, Gaikwad K, Khanna-Chopra R, Singh N (2012) Genomic association for drought tolerance on short arm of wheat chromosome 4B. Funct Integr Genom 12:447–464
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Liao M, Palta A, Fillery IR (2006) Root characteristics of vigorous wheat improve early nitrogen uptake. Aust J Agric Res 57:1097–1107
Liu X, Li R, Chang X, Jing R (2013) Mapping QTLs for seedling root traits in a doubled haploid wheat population under different water regimes. Euphytica 189:51–66
Lorieux M (2012) MapDisto: fast and efficient computation of genetic linkage maps. Mol Breed 30:1231–1235
MacKey J (1973) The wheat root. In: Sears ER, Sears LMS (eds) Proceedings of the 4th international wheat genetics symposium. University of Missouri Columbia, MO, pp 827–842
Manschadi AM, Hammer GH, Christopher JT, de Voil P (2008) Genotypic variation in seedling root architectural traits and implication for drought adaptation in wheat (Triticum aestivum L.). Plant Soil 303:115–129
Manske GGB, Vlek PLG (2002) Root architecture—wheat as a model plant. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half, 3rd edn. Marcel Dekker, New York, pp 249–259
Mergoum M, Harilal VE, Simsek S, Alamri MS, Schatz BG, Kianian SF, Elias E, Kumar A, Bassi FM (2013) Agronomic and quatity QTL mapping in spring wheat. J Plant Breed Genet 1:19–33
Monyo JH, Whttington WJ (1970) Genetic analysis of root growth in wheat. J Agric Sci 74:329–338
Nakamoto T, Oyanagi A (1994) The direction of growth of seminal roots of Triticum aestivum L. and experimental modification thereof. Ann Bot 73:363–367
O’Brien L (1979) Genetic variability of root growth in wheat (Triticum aestivum L.). Aust J Agric Res 30:587–595
O’Tool JC, Bland WL (1987) Genotypic variation in crop plant root systems. Adv Agron 41:91–145
Pestsova E, Ganal MW, Röder MS (2000) Isolation and mapping of microsatellite markers specific for the D genome of bread wheat. Genome 43:689–697
Quarrie SA, Jones HG (1977) Effects of abscisic acid and water stress on development and morphology of wheat. J Exp Bot 28:192–203
Ravi K, Vadez V, Isobe S, Mir RR, Guo Y, Nigam SN, Gowda MV, Radhakrishnan T, Bertioli DJ, Knapp SJ, Varshney RK (2011) Identification of several small main-effect QTL and a large number of epistatic QTL for drought tolerance related traits in groundnut (Arachis hypogaea L.). Theor Appl Genet 122:1119–1132
Ren Y, He X, Liu D, Li J, Zhao X, Li B, Tong Y, Zhang A, Li Z (2012) Major quantitative loci for seminal root morphology of wheat seedling. Mol Breed 30:139–148
Richards RA (2008) Genetic opportunities to improve cereal root systems for dryland agriculture. Plant Prod Sci 11:12–16
Richards RA, Passioura JB (1981) Seminal root morphology and water use in wheat. II. Genetic variation. Crop Sci 21:253–255
Rodríguez VM, Butrón A, Rady MOA, Soengas P, Revilla P (2014) Identification of quantitative trait loci involved in the response to cold stress in maize (Zea mays L.). Mol Breed 33:363–371
Röder MS, Korzun V, Wendehake K, Plaschke J, Tixier MH, Leroy P, Ganal MW (1998) A microsatellite map of wheat. Genetics 149:2007–2023
Sharma S, Xu SZ, Ehdaie B, Hoops A, Close TJ, Lukaszewski AJ, Waines JG (2011) Dissection of QTL effects for root traits using a chromosome arm-specific mapping population in bread wheat. Theor Appl Genet 122:759–769
Siddique KHM, Belford RK, Tennant D (1990) Root:shoot ratios of old and modern, tall and semi-dwarf wheats in Mediterranean environment. Plant Soil 121:89–98
Song QJ, Fickus EW, Cregan PB (2002) Characteristics of trinucleotide markers in wheat. Theor Appl Genet 104:286–293
Song QJ, Shi JR, Singh S, Fickus EW, Costa JM, Lewis J, Gill BS, Ward R, Cregan PB (2005) Development and mapping of microsatellite (SSR) markers in wheat. Theor Appl Genet 110:550–560
STATISTIX V10 (2013) Analytical Software, Tallahassee, FL
Steel RGD, Torrie JH, Dickey DA (1997) Principles and procedures of statistics: a biometrical approach, 3rd edn. McGraw-Hill, New York
Tanksley SD (1993) Mapping polygenes. Annu Rev Genet 27:205–233
Vadez V (2014) Root hydraulics: the forgotten side of roots in drought adaptation. Field Crops Res 165:15–24
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTL. J Hered 93:77–78
Waines JG, Ehdaie B (2007) Domestication and crop physiology: roots of green-revolution wheat. Ann Bot 100:991–998
Wang H, Inukai Y, Yamauchi A (2006) Root development and nutrient uptake. Crit Rev Plant Sci 25:279–301
Wang RX, Hai L, Zhang XY, You GX, Yan CS, Xiao SH (2009) QTL mapping for grain filling rate and yield-related traits in RILs of the Chinese winter wheat population Heshangmai × Yu8679. Theor Appl Genet 118:313–325
Wang S, Basten CJ, Zeng ZB (2012) Windows QTL Cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh, NC. http://statgen.ncsu.edu/qtlcart/WQTLCart.htm
Watt M, Moosavi S, Cunningham SC, Kirkegaard JA, Rebetzke GJ, Richards RA (2013) A rapid, controlled-environment seedling root screen for wheat correlates well with deep rooting depth at vegetative, but not reproductive, stages at two field sites. Ann Bot 112:447–455
Zadok JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14:415–421
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10681-016-1736-9.
Rights and permissions
About this article
Cite this article
Ehdaie, B., Mohammadi, S.A. & Nouraein, M. QTLs for root traits at mid-tillering and for root and shoot traits at maturity in a RIL population of spring bread wheat grown under well-watered conditions. Euphytica 211, 17–38 (2016). https://doi.org/10.1007/s10681-016-1670-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-016-1670-x