Abstract
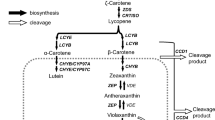
Carotenoid content, composition, and the expression patterns of carotenoid biosynthesis and cleavage genes during petal development were compared among a yellow-flowered deciduous azalea (Rhododendron japonicum f. flavum), a white-flowered evergreen azalea (‘Miyamasatsuki’), and their progeny, to determine the factors that cause reduction in carotenoid content as the petals of the progeny develop. During the early, green petal flowering stage, total carotenoid contents were 31.27 μg g−1 Fresh Weight (F. W.) in R. japonicum f. flavum, 17.84 μg g−1 F. W. in ‘Miyamasatsuki’, and 42.18 μg g−1 F. W. in their progeny. During subsequent flower development, total carotenoid contents remained similar to the green petal stage for R. japonicum f. flavum. However, the content decreased in ‘Miyamasatsuki’ and their progeny at one day before anthesis, and became less than 3 μg g−1 F. W. during the later stages. The expression levels of PSY and PDS increased significantly in R. japonicum f. flavum than in ‘Miyamasatsuki’ as the flowers developed. Their expressions in the progeny were mid-way between both parents. The expression level of CCD4 was significantly higher in ‘Miyamasatsuki’ and the progeny than in R. japonicum f. flavum for all development stages. This result suggested that the high expression level of CCD4, which was inherited from ‘Miyamasatsuki’, was the main factor controlling the reduction in carotenoid content in the progeny.







Similar content being viewed by others
References
Adami M, De Franceschi P, Brandi F, Liverani A, Giovannini D, Rosati C, Dondini L, Tartarini S (2013) Identifying a carotenoid cleavage dioxygenase (ccd4) gene controlling yellow/white fruit flesh colour of peach. Plant Mol Biol Rep 31:1166–1175
Auldridge ME, McCarty DR, Klee HJ (2006) Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr Opin Plant Biol 9:315–321
Chiou CY, Pan HA, Chuang YN, Yeh KW (2010) Differential expression of carotenoid-related genes determines diversified carotenoid colouration in floral tissues of Oncidium cultivars. Planta 232:937–948
Comar CL, Zscheile FP (1942) Analysis of plant extracts for chlorophylls a and b by a photoelectric spectrophotometric method. Plant Physiol 17:198–209
Cunningham FX, Gantt E (1998) Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Phys 49:557–583
Davies BH (1976) The Carotenoids. In: Goodwin TW (ed) The chemistry and biochemistry of plant pigments, vol 2. Academic Press, London, pp 38–165
De Keyser E, De Riek J, Van Bockstaele E (2007) Gene expression profiling of key enzymes in azalea flower colour biosynthesis. Acta Hortic 743:115–120
Fraser PD, Truesdale MR, Bird CR, Schuch W, Bramley PM (1994) Carotenoid biosynthesis during tomato fruit development. Plant Physiol 105:405–413
Galle FC (1985) Deciduous azaleas. In: Galle FC (ed) azalea. Timber Press, Portland, pp 63–116
Giuliano G, Bartley GE, Scolnik PA (1993) Regulation of carotenoid biosynthesis during tomato development. Plant Cell 5:379–387
Goodwin TW, Britton G (1988) Distribution and analysis of carotenoids. In: Goodwin TW (ed) Plant pigments. Academic Press, London, pp 62–132
Hai NTL, Masuda J, Miyajima I, Thien NQ, Mojtahedi N, Hiramatsu M, Kim JH, Okubo H (2012) Involvement of carotenoid cleavage dioxygenase 4 gene in tepal colour change in Lilium brownii var. colchesteri. J Japan Soc Hort Sci 81:366–373
Heursel J (1981) Diversity of flower colours in Rhododendron simsii Planch. and prospects for breeding. Euphytica 30:9–14
Hirschberg J (2001) Carotenoid biosynthesis in flowering plants. Curr Opin Plant Biol 4:210–218
Howitt CA, Pogson BJ (2006) Carotenoid accumulation and function in seeds and non-green tissues. Plant Cell Environ 29:435–445
Huang FC, Molnar P, Schwab W (2009) Cloning and functional characterization of carotenoid cleavage dioxygenase 4 genes. J Exp Bo. 60:3011–3022
Kato M, Ikoma Y, Matsumoto H, Sugiura M, Hyodo H, Yano M (2004) Accumulation of carotenoids and expression of carotenoid biosynthetic genes during maturation in citrus fruit. Plant Physiol 134:824–837
Kato M, Matsumoto H, Ikoma Y, Okuda H, Yano M (2006) The role of carotenoid cleavage dioxygenases in the regulation of carotenoid profiles during maturation in citrus fruit. J Exp Bot 57:2153–2164
Kishimoto S, Ohmiya A (2006) Regulation of carotenoid biosynthesis in petals and leaves of chrysanthemum (Chrysanthemum morifolium). Physiol Plant 128:436–447
Kita M, Kato M, Ban Y, Honda C, Yaegashi H, Ikoma Y, Moriguchi T (2007) Carotenoid accumulation in Japanese Apricot (Prunus mume Siebold & Zucc.): molecular analysis of carotenogenic gene expression and ethylene regulation. J Agric Food Chem 55:3414–3420
Kloer DP, Schulz GE (2006) Structural and biological aspects of carotenoid cleavage. Cell Mol Life Sci 63:2291–2303
Miyajima I, Ureshino K, Kobayashi N, Akabane M (2000) Flower colour and pigments of intersubgeneric hybrid between white-flowered evergreen and yellow-flowered deciduous azaleas. J Japan Soc Hort Sci 69:280–282
Moehs CP, Tian L, Osteryoung KW, DellaPenna D (2001) Analysis of carotenoid biosynthetic gene expression during marigold petal development. Plant Mol Biol 45:281–293
Nakatsuka A, Mizuta D, Kii Y, Miyajima I, Kobayashi N (2008) Isolation and expression analysis of flavonoid biosynthesis genes in evergreen azalea. Sci Hortic 118:314–320
Nakayama M, Miyasaka M, Maoka T, Yagi M, Fukuta N (2006) A Carotenoid-derived yellow Eustoma screened under blue and ultraviolet lights. J Japan Soc Hort Sci 75:161–165
Noguchi Y (1932) Studies on the species crosses of Japanese Rhododendron. I. On the crossability between various species and the cotyledon colour of F1 seedlings. Japan J Bot 6:103–124
Ohmiya A (2009) Carotenoid cleavage dioxygenases and their apocarotenoid products in plants. Plant Biotech 26:351–358
Ohmiya A (2013) Qualitative and quantitative control of carotenoid accumulation in flower petals. Sci Hortic 163:10–19
Ohmiya A, Kishimoto S, Aida R, Yoshioka S, Sumitomo K (2006) Carotenoid cleavage dioxygenase (CmCCD4a) contributes to white colour formation in chrysanthemum petals. Plant Physiol 142:1193–1201
Santamour FS Jr, Dumuth P (1978) Carotenoid flower pigments in Rhododendron. HortScience 13:461–462
Simkin AJ, Schwartz SH, Auldridge M, Taylor MG, Klee HJ (2004) The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles β-ionone, pseudoionone, and geranylacetone. Plant J 40:882–892
Spathmann W (1980) Flavonoids and carotenoids of Rhododendron flowers and their significance for the classification of Rhododendron. In: Luteyn JL, O’Brien ME (eds) Contributions toward a classification of rhododendron s. The New York Botanical Garden, New York, pp 247–275
Ureshino K, Miyajima I, Akabane M (1998) Effectiveness of three-way crossing for the breeding of yellow-flowered evergreen azalea. Euphytica 104:113–118
Vandesompele J, Preter KD, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:1–12
Vishnevetsky M, Ovadis M, Vaintein A (1999) Carotenoid sequestration in plants: the role of carotenoid associated proteins. Trends Plant Sci 4:232–235
Yamagishi M, Kishimoto S, Nakayama M (2010) Carotenoid composition and changes in expression of carotenoid biosynthetic genes in tepals of Asiatic hybrid lily. Plant Breed 129:100–107
Yamaguchi S (1986) In-vitro culture of remote hybrid seedlings aiming to breed new yellow flowered evergreen azalea. Plant Cell Incompat Newsl 18:50–51
Yamamizo C, Kishimoto S, Ohmiya A (2010) Carotenoid composition and carotenogenic gene expression during Ipomoea petal development. J Exp Bot 61:709–719
Yamazaki T (1996) A revision of the genus Rhododendron in Japan, Taiwan, Korea and Sakhalin. Kokusai Bunken Insatsusha, Tokyo
Zhu C, Yamamura S, Koiwa H, Nishihara M, Sandmann G (2002) cDNA cloning and expression of carotenogenic genes during flower development in Gentiana lutea. Plant Mol Biol 48:277–285
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (C) (No. 24580047) from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ureshino, K., Nakayama, M. & Miyajima, I. Contribution made by the carotenoid cleavage dioxygenase 4 gene to yellow colour fade in azalea petals. Euphytica 207, 401–417 (2016). https://doi.org/10.1007/s10681-015-1557-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-015-1557-2