Abstract
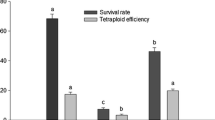
The efficiency of in vitro polyploidization depends on several variables associated to the plant, the antimicrotubule agent and the interactions between them. In the present work, we have used response-surface methodology to determine the best operating conditions for plant recovery in polyploidization assays for shoot apices and somatic embryos of two seedless grape cultivars, employing colchicine and oryzalin. Explant type, tubulin-interfering compound and concentration were the critical factors determining plant recovery. Linear reduction in viable plant regeneration via organogenesis and somatic embryogenesis was obtained by increasing oryzalin concentrations and treatment time, whereas the effects of colchicine were better described by a quadratic design for both explants types. The conditions promoting higher rates of plant recovery were used in chromosome doubling experiments with oryzalin and colchicine for shoot apices and somatic embryos of ‘Crimson seedless’ and ‘BRS Clara’. The established protocols allowed the recovery of non-chimerical autotetraploid plants at rates higher than 30 % for both cultivars. Stomata size parameters statistically correlate to the ploidy level of the regenerants and were effective for preliminary polyploidy screening. Autotetraploid lines of seedless grapes were incorporated into the Vitis germplasm bank for further agronomical evaluations. To our knowledge, this is the first report of in vitro oryzalin induced polyploidization of grapevine and of the use of mathematical modeling to optimize chromosome doubling in plants.






Similar content being viewed by others
References
Alberch P, Gale EA (1983) Size dependence during the development of the amphibian foot. Colchicine-induced digital loss, reduction. J Emb Exp Morphol 76:177–197
Anthony RG, Hussey PJ (1999) Dinitroaniline herbicide resistance and the microtubule cytoskeleton. Trends Plant Sci 4:112–116. doi:10.1016/S1360-1385(99)01378-3
Arora PK, Jyot G, Singh B, Battu RS, Singh B, Aulakh PS (2009) Persistence of imidacloprid on grape leaves, grape berries and soil. Bull Environ Contam Toxicol 82:239–242. doi:10.1007/s00128-008-9554-y
Aversano R, Caruso I, Aronne G, De Micco V, Scognamiglio N, Carputo D (2013) Stochastic changes affect Solanum wild species following autopolyploidization. J Exp Bot 64:625–635. doi:10.1093/jxb/ers357
Beaulieu JM, Leitch IJ, Patel S, Pendharkar A, Knight CA (2008) Genome size is a strong predictor of cell size and stomatal density in angiosperms. New Phytol 179:975–986. doi:10.1111/j.1469-8137.2008.02528.x
Berenschot AS, Zucchi MI, Tulmann-Neto A, Quecini V (2008) Mutagenesis in Petunia × hybrida Vilm. and isolation of a novel morphological mutant. Braz J Plant Physiol 20:95–103. doi:10.1590/S1677-04202008000200002
Bergamini C, Cardone MF, Anaclerio A, Perniola R, Pichierri A, Genghi R, Alba V, Forleo LR, Caputo AR, Montemurro C, Blanco A, Antonacci D (2013) Validation assay of VvAGL11 marker in a wide range of genetic background for early selection of stenospermocarpy in Vitis vinifera L. Mol Biotechnol 54:1021–1030. doi:10.1007/s12033-013-9654-8
Besnard F, Vernoux T, Hamant O (2011) Organogenesis from stem cells in planta: multiple feedback loops integrating molecular and mechanical signals. Cell Mol Life Sci 68:2885–2906. doi:10.1007/s00018-011-0732-4
Breviario D, Gianì S, Morello L (2013) Multiple tubulins: evolutionary aspects and biological implications. Plant J. doi:10.1111/tpj.12243
Cai X, Kang X-Y (2011) In vitro tetraploid induction from leaf explants of Populus pseudo-simonii Kitag. Plant Cell Rep 30:1771–1778. doi:10.1007/s00299-011-1085-z
Camargo UA, Nachtigal JC, Maia JDG, Oliveira PRD, Protas JFS (2003) BRS Clara: nova cultivar de uva de mesa branca sem semente. Bento Gonçalves: Embrapa-CNPUV (Embrapa Uva e Vinho. Comunicado Técnico, 46), in Portuguese. http://www.cnpuv.embrapa.br/publica/comunicado/#a2003. Accessed 10 May 2013
Caperta AD, Delgado M, Ressurreição F, Meister A, Jones RN, Viegas W, Houben A (2006) Colchicine-induced polyploidization depends on tubulin polymerization in c-metaphase cells. Protoplasma 227:147–153. doi:10.1159/000151319
Carvalho JFR, Carvalho CR, Otoni WC (2005) In vitro induction of polyploidy in annatto (Bixa orellana). Plant Cell Tiss Organ Cult 80:69–75. doi:10.1007/s11240-004-8833-5
Chalak L, Legave JM (1996) Oryzalin combined with adventitious regeneration for an efficient chromosomoe doubling of trihaploid kiwifruit. Plant Cell Rep 16:97–100. doi:10.1007/BF01275459
Chatfield SP, Capron R, Severino A, Penttila PA, Alfred S, Nahal H, Provart NJ (2013) Incipient stem cell niche conversion in tissue culture: using a systems approach to probe early events in WUSCHEL-dependent conversion of lateral root primordia into shoot meristems. Plant J 73:798–813. doi:10.1111/tpj.12085
Chen ZJ (2010) Molecular mechanisms of polyploidy and hybrid vigor. Trend Plant Sci 15:57–71. doi:10.1016/j.tplants.2009.12.003
Dermen H (1954) Colchiploidy in grapes. J Hered 45:159–172
Dhooghe E, Van Laere K, Eeckhaut T, Leus L, Van Huylenbroeck J (2011) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tiss Organ Cult 104:359–373. doi:10.1007/s11240-010-9786-5
Dokoozlian N, Luvisi D, Moriyama M, Schrader P (1995) Cultural practices improve color, size of ‘Crimson Seedless’. California Agric 49:36–40. doi:10.3733/ca.v049n02p36
Doyle JJ, Flagel LE, Paterson AH, Rapp RA, Soltis DE, Soltis PS, Wendel JF (2008) Evolutionary genetics of genome merger and doubling in plants. Annu Rev Genet 42:443–461. doi:10.1146/annurev.genet.42
DuClercq J, Sangwan-Norreel B, Catterou M, Sangwan RS (2011) De novo shoot organogenesis: from art to science. Trends Plant Sci 16:597–606. doi:10.1016/j.tplants.2011.08.004
Dutt M, Li ZT, Dhekney SA, Gray DJ (2008) Transgenic plants from shoot apical meristems of Vitis vinifera L. “Thompson Seedless” via Agrobacterium-mediated transformation. Plant Cell Rep 26:2101–2110. doi:10.1007/s00299-007-0424-6
Franks T, Gang He D, Thomas MR (1998) Regeneration of transgenic shape Vitis vinifera L. sultana plants: genotypic and phenotypic analysis. Mol Breed 4:321–333. doi:10.1023/A:1009673619456
Galzy R (1964) Technique de thermotherapie des viroses de la vigne. Ann Epiphyt 15:245–256
Gambino G, Minuto M, Boccacci P, Perrone I, Vallania R, Gribaudo I (2011) Characterization of expression dynamics of WOX homeodomain transcription factors during somatic embryogenesis in Vitis vinifera. J Exp Bot 62:1089–1101. doi:10.1093/jxb/erq349
Gray DJ, Fisher LC (1985) In vitro shoot propagation of grape species, hybrids and cultivars. Proc Fla State Hort Soc 98:172–174
Gribaudo I, Gambino G, Vallania R (2004) Somatic embryogenesis from grapevine anthers: identification of the optimal developmental stage for collecting explants. Am J Enol Vitic 55:427–430
Häntzschel KR, Weber G (2010) Blockage of mitosis in maize root tips using colchicine-alternatives. Protoplasma 241:99–104. doi:10.1007/s00709-009-0103-2
Hodgson JG, Sharafi M, Jalili A, Díaz S, Montserrat-Martí G, Palmer C, Cerabolini B, Pierce S, Hamzehee B, Asri Y, Jamzad Z, Wilson P, Raven JA, Band SR, Basconcelo S, Bogard A, Carter G, Charles M, Castro-Díez P, Cornelissen JH, Funes G, Jones G, Khoshnevis M, Pérez-Harguindeguy N, Pérez-Rontomé MC, Shirvany FA, Vendramini F, Yazdani S, Abbas-Azimi R, Boustani S, Dehghan M, Guerrero-Campo J, Hynd A, Kowsary E, Kazemi-Saeed F, Siavash B, Villar-Salvador P, Craigie R, Naqinezhad A, Romo-Díez A, de Torres Espuny L, Simmons E (2010) Stomatal vs. genome size in angiosperms: the somatic tail wagging the genomic dog? Ann Bot 105:573–584. doi:10.1093/aob/mcq011
Jürgens G, Mayer U, Torres Ruiz RA, Berleth T, Misera S (1991) Genetic analysis of pattern formation in Arabidopsis embryo. Dev Suppl 1:27–38
Kasha KJ (2005) Chromosome doubling and recovery of doubled haploid plants. Haploids in crop improvement II, vol 56. Springer, Berlin, pp 123–152
Koch EA, Spitzer RH (1982) Autoradiographic studies of protein, polysaccharide synthesis during vitellogenesis in Drosophila. Cell Tiss Res 224:315–333
Koch EA, Spitzer RH (1983) Multiple effects of colchicine on oogenesis in Drosophila, induced sterility, switch of potential oocyte to nurse-cell developmental pathway. Cell Tiss Res 228:21–32
Kuliev VM (2011) Induced autotetraploid grape mutants. Cytol Genet 45:163–169
Langhans M, Niemes S, Pimpl P, Robinson DG (2009) Oryzalin bodies: in addition to its anti-microtubule properties, the dinitroaniline herbicide oryzalin causes nodulation of the endoplasmic reticulum. Protoplasma 236:73–84. doi:10.1007/s00709-009-0059-2
Laux T, Mayer KF, Berger J, Jurgens G (1996) The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122:87–96
Lenth RV (2009) Response-surface methods in R using rsm. J Stat Soft 32:1–17. doi: http://www.jstatsoft.org/v32/i07/. Accessed 22 Aug 2013
Liu XZ, Lin H, Mo XY, Long T, Zhang HY (2009) Genetic variation in colchicine-treated regenerated plants of Eucalyptus globulus Labill. J Genet 88:345–348
López-Miranda S, Hernández-Sánchez P, Serrano-Martínez A, Hellín P, Fenoll J, Núñez-Delicado E (2011) Effect of ripening on protein content and enzymatic activity of crimson seedless table grape. Food Chem 127:481–486. doi:10.1016/j.foodchem.2011.01.027
Luckett D (1989) Colchicine mutagenesis is associated with substantial heritable variation in cotton. Euphytica 42:177–182. doi:10.1007/BF00042630
Maillot P, Lebel S, Schellenbaum P, Jacques A, Walter B (2009) Differential regulation of SERK, LEC1-like and pathogenesis-related genes during indirect secondary somatic embryogenesis in grapevine. Plant Physiol Biochem 47:743–752. doi:10.1016/j.plaphy.2009.03.016
Marsoni M, Bracale M, Espen L, Prinsi B, Negri AS, Vannini C (2008) Proteomic analysis of somatic embryogenesis in Vitis vinifera. Plant Cell Rep 27:347–356. doi:10.1007/s00299-007-0438-0
Martinelli L, Gribaudo I (2009) Strategies for effective somatic embryogenesis in grapevine: an appraisal. In: Angelakis-Roubelakis KA (ed) Grapevine molecular physiology & biotechnology. Springer, Dordercht, pp 461–493. doi:10.1007/978-90-481-2305-6_17
Motosugi H, Okudo K, Kataoka D, Naruo T (2002) Comparison of growth characteristics between diploid and colchicine-induced tetraploid grapevine rootstocks. J Jpn Soc Hort Sci 71:335–341
Nitsch JP, Pratt C, Nitsch C, Shaulis NJ (1960) Substances in concord and concord seedless grapes in relation to berry development. Am J Bot 47:566–576
Notsuka K, Tsuru T, Shiraishi M (2000) Induced polyploid grapes via in vitro chromosome doubling. J Jpn Soc Hortic Sci 69:543–551
Olmo HP (1952) Breeding tetraploid grapes. Proc Am Soc Hort Sci 59:285–290
Parisod C, Holderegger R, Brochmann C (2010) Evolutionary consequences of autopolyploidy. New Phytol 186:5–17. doi:10.1111/j.1469-8137.2009.03142.x
Parrotta L, Cai G, Cresti M (2010) Changes in the accumulation of alpha- and beta-tubulin during bud development in Vitis vinifera L. Planta 231:277–291. doi:10.1007/s00425-009-1053-9
Poloz Y, O’Day DH (2012) Colchicine affects cell motility, pattern formation and stalk cell differentiation in Dictyostelium by altering calcium signaling. Differentiation 83:185–199. doi:10.1016/j.diff.2011.12.006
Predieri S (2001) Mutation induced and tissue culture in improving fruits. Plant Cell Tiss Org Cult 64:185–210. doi:10.1023/A:1010623203554
R Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, ISBN 3-900051-07-0. http://www.R-project.org/. Accessed 23 Aug 2013
Ramming DW, Tarailo R, Badr SA (1995) ‘Crimson Seedless’: a new late maturing, red seedless table grape. HortScience 30:1473–1474
Reed BM, Wada S, DeNoma J, Niedz RP (2013) Improving in vitro mineral nutrition for diverse pear germplasm. In Vitro Cell Dev Biol 49:343–355. doi:10.1007/s11627-013-9504-1
Rêgo MM, Rêgo ER, Bruckner CH, Finger FL, Otoni WC (2011) In vitro induction of autotetraploids from diploid yellow passion fruit mediated by colchicine and oryzalin. Plant Cell Tiss Organ Cult 107:451–459. doi:10.1007/s11240-011-9995-6
Sarikani H, Wakana A (2009) Effect of ploidy on parthenocarpy in grape cultivars. Acta Horticult 827:433–438. http://www.actahort.org/books/827/827_74.htm. Accessed 10 May 2013
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. doi:10.1038/nmeth.2089
Sharma D, Awasthi MD (2003) Behaviour of forchlorfenuron residues in grape, soil and water. Chemosphere 50:589–594. doi:10.1016/S0045-6535(02)00619-7
Shiraishi M, Fujishima H, Chijiwa H (2008) Tetraploid sucrose-accumulating grapevines. Vitis 47:191–192
Shiraishi M, Fujishima H, Chijiwa H (2010) Evaluation of table grape genetic resources for sugar, organic acid, and amino acid composition of berries. Euphytica 174:1–13. doi:10.1007/s10681-009-0084-4
Shiraishi M, Fujishima H, Chijiwa H, Muramoto K (2012) Estimates of genotypic and yearly variations on fruit quality and functional traits for tetraploid table grape breeding. Euphytica 185:243–251. doi:10.1007/s10681-011-0562-3
Stout AB (1936) Seedlessness in grapes.Technical Bulletin, N. 238. New York State Agricultural Experiment Station, Geneva
Sweetman C, Wong DC, Ford CM, Drew DP (2012) Transcriptome analysis at four developmental stages of grape berry (Vitis vinifera cv. Shiraz) provides insights into regulated and coordinated gene expression. BMC Genomics 13:691. doi:10.1186/1471-2164-13-691
Vainola A (2000) Polyploidization and early screening of Rhododendron hybrids. Euphytica 112:239–244. doi:10.1023/A:1003994800440
Van Tuyl JM, Meijer B, Van Dien MP (1992) The use of oryzalin as an alternative for colchicines in vitro chromosome doubling of Lilium and Nerina. Acta Hortic 325:625–630
Wada S, Niedz RP, DeNoma J, Reed BM (2013) Mesos components (CaCl2, MgSO4, KH2PO4) are critical for improving pear micropropagation. In Vitro Cell Dev Biol Plant 49:356–365. doi:10.1007/s11627-013-9508-x
Wakana A, Park SM, Hiramatsu M, Iianada N, Fukudome I, Yasukochi K (2005) Characteristics of seedless berries of tetraploid hybrid grapes (Vitis complex) from reciprocal crosses between diploid ‘Muscat Bailey A’ and tetraploid ‘Red Pearl’. J Fac Agr Kyushu Uni 50:49–59
Xue J, Wang S, You X, Dong J, Han L, Liu F (2011) Multi-residue determination of plant growth regulators in apples and tomatoes by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 25:3289–3297. doi:10.1002/rcm.5225
Yang XM, Cao ZY, An LZ, Wang YM, Fang XW (2006) In vitro tetraploid induction via colchicine treatment from diploid somatic embryos in grapevine (Vitis vinifera L.). Euphytica 152:217–224. doi:10.1007/s10681-006-9203-7
Yu Z, Haage K, Streit VE, Gierl A, Torres-Ruiz RA (2009) A large number of tetraploid Arabidopsis thaliana lines, generated by a rapid strategy, reveal high stability of neo-tetraploids during consecutive generations. Theor App Gen 118:1107–1119. doi:10.1007/s00122-009-0966-9
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sinski, I., Dal Bosco, D., Pierozzi, N.I. et al. Improving in vitro induction of autopolyploidy in grapevine seedless cultivars. Euphytica 196, 299–311 (2014). https://doi.org/10.1007/s10681-013-1034-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-013-1034-8