Abstract
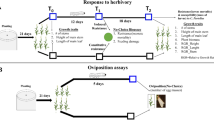
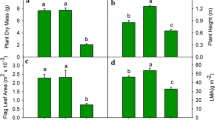
Striga hermonthica (Del.) Benth. is a parasitic weed on tropical cereals causing serious yield losses in Africa. The use of host crop varieties with improved resistance and tolerance against this parasite is a key component of an integrated control strategy. Breeding for tolerance is however seriously hampered by the absence of reliable and yet practical selection measures. The observation that the photosynthetic rate of tolerant genotypes is less sensitive to Striga infection was used as a starting point to search for suitable selection measures. In a greenhouse pot experiment the effect of Striga infection on the photosynthesis of four sorghum (Sorghum bicolor [L.] Moench) genotypes, differing in Striga tolerance level, was measured at three moments in time (26, 48 and 75 days after sowing). Genotypes were CK60-B, E36-1, Framida and Tiémarifing. Measurements involved CO2-assimilation (A) and three chlorophyll fluorescence characteristics (electron transport rate through photosystem II [ETR], photochemical [Pq] and non-photochemical quenching [NPq]). Striga infection negatively affected A, ETR and Pq. Based on A and Pq, genotypes with superior levels of tolerance (Tiémarifing) could be discriminated from genotypes with superior level of resistance (Framida). Both A and Pq showed high heritabilities and consequently clear and predictable differences between genotypes. Using discriminative ability, heritability and cost effectiveness as main criteria, photochemical quenching (Pq) was concluded to possess the highest potential to serve as indirect selection measure for host plant tolerance to Striga. Screening should preferably be conducted at relatively high Striga infestation levels, between Striga emergence and host plant flowering.


Similar content being viewed by others
Abbreviations
- A :
-
Leaf CO2-assimilation rate (μmol CO2 m−2 s−1)
- ETR :
-
The electron transport rate through photosystem II (μmol m−2 s−1)
- f abs :
-
The absorbtivity of the leaf (−)
- Fm′:
-
The maximum fluorescence emission induced by a saturating light pulse in the light (−)
- Fm :
-
The maximum fluorescence emission induced by a saturating light pulse in the dark (−)
- Fo′:
-
The basic fluorescence in the light when all PSII centres are oxidized by a period of far-red light (−)
- F t :
-
The steady-state fluorescence emission (−)
- I :
-
The light intensity (μmol photon m−2 s−1)
- NPq :
-
The level of non-photochemical quenching (−)
- φ2 :
-
The electron transport efficiency of PSII (−)
- PAR:
-
Photosynthetically active radiation (μmol photon m−2 s−1)
- PS:
-
Photosystem
- Pq :
-
The level of photochemical quenching (−)
- R :
-
Repeatability
- RYL :
-
Relative yield loss (%)
- S 2 :
-
Within-group variance component
- S 2 A :
-
Among-group variance component
- V EG :
-
General environmental variance
- V ES :
-
Environmental variance due to temporary or localized environmental effects
- V G :
-
Genotypic variance
- V P :
-
Phenotypic variance
References
Ackroyd RD, Graves JD (1997) The regulation of the water potential gradient in the host and parasite relationship between Sorghum bicolor and Striga hermonthica. Ann Bot 80:649–656
Arnaud MC, Renaudin S, Villaine A, Thalouarn P (1996) Why is sorghum bicolor var Framida resistant to Striga hermonthica? A histological and molecular approach. In: Moreno MT, Cubero JI, Berner D, Joel D, Musselman LJ, Parker C (eds) Advances in parasitic plant research. Sixth International Parasitic Weed Symposium, Cordoba , pp 574–580
Bebawi FF, Farah AF (1981) Effects of parasitic and non-parasitic weeds on sorghum. Exp Agri 17:415–418
Becker, WA (1984) A manual of quantitative genetics. Academic Enterprises, Pullman, Washington
Björkman O, Powles SP (1984) Inhibition of photosynthetic reactions under water stress: interaction with light level. Planta 153:376–387
DeVries J (2000) The inheritance of Striga reactions in maize. In: Haussmann BIG, Hess DE, Koyama ML, Grivet L, Rattunde HFW, Geiger HH (eds) Breeding for striga resistance in cereals. Margraf Verlag, Weikersheim, Ibadan, pp 73–84
Doggett H (1982) Factors reducing sorghum yields: Striga and birds. ICRISAT, Patancheru, pp 313–320
Drennan DSH, El Hiweris SO (1979) Changes in growth regulator substances in Sorghum vulgare infected with Striga hermonthica. In: Musselman LJ, Worsham AD, Eplee RE (eds) 2nd International Symposium on Parasitic Weeds North Carolina State University, Raleigh
El Hiweris SO (1987) Nature of resistance to Striga hermonthica (Del.) Benth. parasitism in some Sorghum vulgare (Pers.) cultivars. Weed Res 27:305–312
Falconer DS, Mackay TFC (1996) Introduction to quantitative genetics. 4th edn. Longman Group Limited, Essex
FAOSTAT (2004) http://faostat.fao.org/faostat/
Fracheboud Y, Haldimann P, Leipner J, Stamp P (1999) Chlorophyll fluorescence as a selection tool for cold tolerance of photosynthesis in maize (Zea mays L.). J Exp Bot 50:1533–1540
Frost DL, Gurney AL, Press MC, Scholes JD (1997) Striga hermonthica reduces photosynthesis in sorghum: the importance of stomatal limitations and a potential role for ABA. Plant Cell and Environ 20:483–492
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990:87–92
Goudriaan J, Laar HHv (1994) Modelling potential crop growth processes. Kluwer Publishers, Dordrecht
Graves JD, Press MC, Stewart GR (1989) A carbon balance model of the sorghum Striga hermonthica host-parasite association. Plant Cell and Environment 12:101–108
Gurney AL, Press MC, Ransom JK (1995) The parasitic angiosperm Striga hermonthica can reduce photosynthesis of its sorghum and maize hosts in the field. J Exp Bot 46:1817–1823
Gurney AL, Press MC, Scholes JD (1999) Infection time and density influence the response of sorghum to the parasitic angiosperm Striga hermonthica. New Phytologist 143:573–580
Gurney AL, Taylor A, Mbwaga A, Scholes JD, Press MC (2002) Do maize cultivars demonstrate tolerance to the parasitic weed Striga asiatica? Weed Res 42:299–306
Harbinson J (1995) Detection of stress in pot plants. Acta Horticultura 405:320–334
Haussmann BIG, Hess DE, Reddy BVS, Mukuru SZ, Kayentao M, Welz HG, Geiger HH (2001a) Pattern analysis of genotype X environment interaction for Striga resistance and grain yield in African sorghum trials. Euphytica 122:297–308
Haussmann BIG, Hess DE, Reddy BVS, Mukuru SZ, Kayentao M, Welz HG, Geiger HH (2001b) Quantitative-genetic parameters of sorghum growth under Striga infestation in Mali and Kenya. Plant Breed 120:49–56
Havaux M, Lannoye R (1985) Drought resistance of hardy wheat cultivars measured by a rapid chlorophyll fluorescence test. J Agri Sci Cambridge 104:501–504
Kim SK (1991) Breeding for tolerance and general resistance in Maize: a novel approach to combating Striga in Africa. Nairobi, Kenya, pp 168–176
Kling JG, Fajemisin JM, Badu Apraku B, Diallo A, Menkir A, Melake Berhan A (2000) Striga resistance breeding. In: Haussmann BIG, Hess DE, Koyama ML, Grivet L, Rattunde HFW, Geiger HH (eds) Breeding for striga resistance in cereals. Proceedings of a workshop held at IITA, Margraf Verlag, Weikersheim, Ibadan, pp 103–118
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk, pp 116–121
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Nogues S, Alegre L, Araus JL, Perez-Aranda L, Lannoye R (1994) Modulated chlorophyll fluorescence and photosynthetic gas exchange as rapid screening methods for drought tolerance in barley genotypes. Photosynthetica 30:465–474
Okonkwo SNC (1966) Studies on Striga senegalensis II. Translocation of C14 labelled photosynthate, urea-C14 and sulphur-35 between host and parasite. Am J Bot 53:142–148
Olivier A., Ramaiah KV, Leroux GD (1991) Selection of sorghum (Sorghum bicolor (L.) Moench) varieties resistant to the parasitic weed Striga hermonthica (Del.) Benth. Weed Res 31:219–226
Olsovska K, Brestic M, Hudec J (2000) Use of physiological characteristics of barley (Hordeum vulgare L.) for screening genotypes tolerant to drought. Acta Fytotechnica et Zootechnica 3:103-106
Ort DR, Baker NR (2002) A photoprotective role for O2 as an alternative electron sink in photosynthesis? Curr Opin Plant Biol 5:193–198
Oxborough K, Baker NR (1997) Resolving chlorophyll fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components - calculation of qP and Fv/Fm without measuring Fo. Photosynth Res 54:135–142
Pageau K, Simier P, Le Bizec B, Robins RJ, Fer A (2003) Characterization of nitrogen relationships between Sorghum bicolor and the root-hemiparasitic angiosperm Striga hermonthica (Del.) Benth. using K15NO3 as isotopic tracer. J Exp Bot 54:789–799
Parker C, Riches CR (1993) Parasitic weeds of the world: biology and control. Cab International, Wallingford, Oxon
Pierce S, Mbwaga AM, Press MC, Scholes JD (2003) Xenognosin production and tolerance to Striga asiatica infection of high-yielding maize cultivars. Weed Res 43:139–145
Prabhakara Setty TK, Hosmani MM (1981) Effect of Striga infestation of sorghum. In: ICRISAT (ed) Effect of striga infestation of sorghum. Proceedings of the Eight Asian-Pacific Weed Science Society, ICRISAT, Patancheru, India, pp 287–289
Press MC, Shah N, Tuohy JM, Stewart GR (1987a) Carbon isotope ratios demonstrate carbon flux from C4 host to C3 parasite. Plant Physiol 85:1143–1145
Press MC, Stewart GR (1987b) Growth and photosynthesis in sorghum-bicolor infected with Striga hermonthica. Ann Bot 60:657–662
Press MC, Tuohy JM, Stewart GR (1987b) Gas exchange characteristics of the sorghum Striga host-parasite association. Plant Physiol 84:814–819
Rascher U, Liebig M, Lüttge U (2000) Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant Cell Environ 23:1397–1405
Rodenburg J, Bastiaans L, Kropff MJ (2006a) Characterization of host tolerance to Striga hermonthica. Euphytica 147:353–365
Rodenburg J, Bastiaans L, Kropff MJ, Van Ast A (2006b) Effects of host plant genotype and seedbank density on Striga reproduction. Weed Res 46:251–263
Rodenburg J, Bastiaans L, Weltzien E, Hess DE (2005) How can field selection for Striga resistance and tolerance in sorghum be improved? Field Crops Res 93:34–50
Rogers WE, Nelson RR (1962) Penetration and nutrition of Striga asiatica. Phytopathology 52:1064–1070
Sauerborn J (1991) The economic importance of the phytoparasites Orobanche and Striga. In: Ransom JK, Musselman LJ, Worsham AD, Parker C (eds) Proceedings, 5th International Symposium on Parasitic Weeds, CIMMYT, Nairobi, Kenya, pp 137–143
Schafer JF (1971) Tolerance to plant disease. Ann Rev Phytopathol 9:235–252
Schapendonk AHCM, Dolstra O, Kooten Ov (1989a) The use of chlorophyll fluorescence as a screening method for cold tolerance in maize. Photosynth Res 20:235–247
Schapendonk AHCM, Spitters CJT, Groot PJ (1989b) Effects of water stress on photosynthesis and chlorophyll fluoresence of five potato cultivars. Potato Res 32:17–32
Schapendonk AHCM, Putten PELvd, Dolstra O, Haalstra SR, Tonk WJM (1992) Chlorophyll fluorescence: a non-destructive method for detecting damage in the photosynthetic apparatus in plants. Acta Horticultura 304:61–70
Scharen AL, Krupinsky JM (1969) Effect of Septoria nodorum infection on CO2 absorption and yield of wheat. Phytopathology 59:1298–1301
Schreiber U (1986) Detection of rapid induction kinetics with a new type of high-frequency modulated chlorophyll fluorometer. Photosynth Res 9:261–272
Showemimo FA (2003) Selection criteria for combining high yield and Striga resistance in Sorghum. Tropicultura 21:157–159
Smith LH, Keys AJ, Evans MCW (1995) Striga hermonthica decreases photosynthesis in Zea mays through effects on leaf cell structure. J Exp Bot 46:759–765
Taylor A, Martin J, Seel WE (1996) Physiology of the parasitic association between maize and witchweed (Striga hermonthica): is ABA involved. J Exp Bot 47:1057–1065
Vasudeva Rao MJ, Chidley VL, House LR (1989) Estimates of grain yield losses caused in sorghum (Sorghum bicolor L. Moench) by Striga asiatica (L.) Kuntze obtained using the regression approach. Agri Ecosyst Environ 25:139–150
White PC, Wilson GL (1965) Effects of water stress on the reproductive development of sorghum vulgare pers. University of Queensland papers IV
Acknowledgements
Financial assistance for this study was made possible through the beneficence of the Netherlands Foundation for the Advancement of Tropical Research (WOTRO). We thank Wageningen University (WU), Wageningen Plants Sciences Experimental Center (WPSEC) and the Africa Rice Center (WARDA) and in particular Ans Hofman and Henriette Drenth (WU), Geurt Versteeg, Peter Saat, Taede Stoker and Henk Meurs (WPSEC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodenburg, J., Bastiaans, L., Schapendonk, A.H.C.M. et al. CO2-assimilation and chlorophyll fluorescence as indirect selection criteria for host tolerance against Striga . Euphytica 160, 75–87 (2008). https://doi.org/10.1007/s10681-007-9555-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-007-9555-7