Abstract
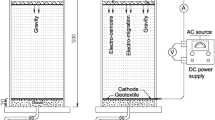
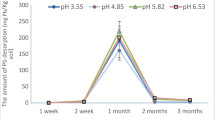
This is the second paper of two companion papers presenting the results of laboratory bench-scale experimental studies on electrokinetic extraction of lead from two different kaolinites. The theoretical formulation and numerical simulation of the process are presented in the first paper. Two different kaolinites were used in the study: (1) Georgia kaolinite and (2) Milwhite kaolinite. The lead spiked in Georgia kaolinite was highly mobilized and effectively extracted by the technique as the pH in the soil was significantly lowered by the electrokinetic process. Milwhite kaolinite has a much higher acid/base buffer capacity, and the required acidic environment could not be developed. As a result, removal of the lead spiked in Milwhite kaolinite was minimal. Comparison between simulations and experimental results is also presented. Factors affecting the cleanup efficiency of the process and potential enhancement techniques are also discussed.






Similar content being viewed by others
References
Acar YB, Gale RJ, Putnam GA, Hamed J, Wong RL (1990) Electrochemical processing of soils: Theory of pH gradient development by diffusion, migration, and linear convection. J Environ Sci Health Part A—Environ Sci Eng Toxic Hazard Subst Control 25(6):687–714
Acar YB, Li H, Gale RJ (1992) Phenol removal from kaolinite by electrokinetics. ASCE J Geotech Eng 118(11):1837–1852
Banerjee S, Horng JI, Ferguson JF (1991) Field experience with electrokinetics at a superfund site. In: Energy and environmental issues. Transp Res Rec 1312, Transp Res Board, Washington, DC, pp 167–174
Bruell CJ, Segall BA, Walsh MT (1992) Electroosmotic removal of gasoline hydrocarbons and TCE from clay. ASCE J Environ Eng 118(1):68–83
Cox CD, Shoesmith MA, Ghosh MM (1996) Electrokinetic remediation of mercury-contaminated soils using iodine/iodide lixiviant. Environ Sci Technol 30(6):1933–1938
Ellis WD, Fogg TR, Tafuri AN (1986) Treatment of soils contaminated with metals. In: Proceedings of 12th Annual Research Symposium: Land disposal, remedial action, incineration and treatment of hazardous waste, Report no. EPA/600/S9-86/022, US EPA, Cincinnati, pp 201–207
Eykholt GR, Daniel DE (1994) Impact of system chemistry on electroosmosis in contaminated soil. ASCE J Geotech Eng 120(5):797–815
Gopinath S (1994) A laboratory study of electro-kinetic remediation of fine-grained soil contaminated with organic compounds. MS Thesis, Texas A&M University
Gu Y-Y, Yeung AT, Li H-J (2009a) EDTA-enhanced electrokinetic extraction of cadmium from a natural clay of high buffer capacity. In: Proceedings of International Symposium on Geoenvironmental Engineering (ISGE 2009), Hangzhou, pp 790–795
Gu Y-Y, Yeung AT, Koenig A, Li H (2009b) Effects of chelating agents on the zeta potential of cadmium-contaminated natural clay. Sep Sci Technol 44(10):2203–2222
Hamed J, Acar YB, Gale RJ (1991) Pb(II) removal from kaolinite using electrokinetics. ASCE J Geotech Eng 117(2):241–271
Ho SV, Athmer C, Sheridan PW, Hughes BM, Orth R, Mckenzie D, Brodsky PH, Shapiro A, Thornton R, Salvo J, Schultz D, Landis R, Griffith R, Shoemaker S (1999a) The Lasagna technology for in situ soil remediation. 1. Small field test. Environ Sci Technol 33(7):1086–1091
Ho SV, Athmer C, Sheridan PW, Hughes BM, Orth R, Mckenzie D, Brodsky PH, Shapiro AM, Sivavec TM, Salvo J, Schultz D, Landis R, Griffith R, Shoemaker S (1999b) The Lasagna technology for in situ soil remediation. 2. Large field test. Environ Sci Technol 33(7):1092–1099
Lageman R, Godschalk MS (2007) Electro-bioreclamation—A combination of in situ remediation techniques proves successful at a site in Zeist, the Netherlands. Electrochim Acta 52(10):3449–3453
Lageman R, Clarke RL, Pool W (2005) Electro-reclamation, a versatile soil remediation solution. Eng Geol 77(3–4):191–201
Lee HH, Yang JW (2000) A new method to control electrolytes pH by circulation system in electrokinetic soil remediation. J Hazard Mater 77(1–3):227–240
Leinz RW, Hoover DB, Meier AL (1998) NEOCHIM: an electrochemical method for environmental application. J Geochem Explor 64(1–3):421–434
Maini G, Sharman AK, Knowles CJ, Sunderland G, Jackman SA (2000) Electrokinetic remediation of metals and organics from historically contaminated soil. J Chem Technol Biotechnol 75(8):657–664
Menon RM (1996) Numerical modeling and experimental study on electro-kinetic extraction. Dissertation, Texas A&M University
Pamukcu S, Wittle JK (1992) Electrokinetic removal of selected heavy metals from soil. AIChE Environ Prog 11(3):241–250
Probstein RF, Hicks RE (1993) Removal of contaminants from soils by electric fields. Sci 260(5107):498–503
Reddy KR, Chaparro C, Saichek RE (2003) Iodide-enhanced electrokinetic remediation of mercury-contaminated soils. ASCE J Environ Eng 129(12):1137–1148
Rødsand T, Acar YB, Breedveld G (1995) Electrokinetic extraction of lead from spiked Norwegian marine clay. In: Acar YB, Daniel DE (eds) Characterization, containment, remediation, and performance in environmental geotechnics. Geotech Spec Publ No 46, vol 2. ASCE, New York, pp 1518–1534
Sawada A, Tanaka S, Fukushima M, Tatsumi K (2003) Electrokinetic remediation of clayey soils containing copper(II)-oxinate using humic acid as a surfactant. J Hazard Mater 96(2–3):145–154
Shapiro AP, Probstein RF (1993) Removal of contaminants from saturated clay by electroosmosis. Environ Sci Technol 27(2):283–291
Suèr P, Allard B (2003) Mercury transport and speciation during electrokinetic soil remediation. Water Air Soil Pollut 143(1–4):99–109
van Olphen H (1977) An introduction to clay colloid chemistry, 2nd edn. Wiley, New York
van Olphen H, Fripiat JJ (eds) (1979) Data summaries—Clay minerals society. In: Data handbook for clay materials and other non-metallic minerals. Pergamon Press, New York, pp 13–37
Yang GCC, Long Y-W (1999) Removal and degradation of phenol in a saturated flow by in situ electrokinetic remediation and Fenton-like process. J Hazard Mater 69(3):259–271
Yeung AT (1992) Diffuse double-layer equations in SI units. ASCE J Geotech Eng 118(12):2000–2005
Yeung AT (1994) Electrokinetic flow processes in porous media and their applications. In: Corapcioglu MY (ed) Advances in porous media, vol 2. Elsevier, Amsterdam, pp 309–395
Yeung AT (2006a) Contaminant extractability by electrokinetics. Environ Eng Sci 23(1):202–224
Yeung AT (2006b) Fundamental aspects of prolonged electrokinetic flows in kaolinites. Geomech and Geoeng: An Int J 1(1):13–25
Yeung AT (2008) Electrokinetics for soil remediation. In: Yeung AT, Lo IMC (eds) Environmental geotechnology and global sustainable development 2008. Advanced Technovation Ltd, Hong Kong, pp 16–25
Yeung AT (2009a) Remediation technologies for contaminated sites. In: Proceedings of the International Symposium on Geoenvironmental Engineering (ISGE 2009), Hangzhou, pp 328–369
Yeung AT (2009b) Geochemical processes affecting electrochemical remediation. In: Reddy K, Cameselle C (eds) Electrochemical remediation technologies for polluted soils, sediments and groundwater. Wiley, New York, pp 65–94
Yeung AT, Datla S (1995) Fundamental formulation of electrokinetic extraction of contaminants from soil. Can Geotech J 32(4):569–583
Yeung AT, Hsu C (2005) Electrokinetic remediation of cadmium-contaminated clay. ASCE J Environ Eng 131(2):298–304
Yeung AT, Hsu C, Menon RM (1996) EDTA-enhanced electrokinetic extraction of lead. ASCE J Geotech Eng 122(8):666–673
Yeung AT, Hsu C, Menon RM (1997a) Physicochemical soil-contaminant interactions during electrokinetic extraction. J Hazard Mater 55(1–3):221–237
Yeung AT, Scott TB, Gopinath S, Menon RM, Hsu C (1997b) Design, fabrication, and assembly of an apparatus for electrokinetic remediation studies. ASTM Geotech Test J 20(2):199–210
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsu, Cn., Yeung, A.T. & Menon, R.M. Electrokinetic extraction of lead from kaolinites: II. Experimental investigation. Environmentalist 31, 33–38 (2011). https://doi.org/10.1007/s10669-010-9298-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10669-010-9298-1