Abstract
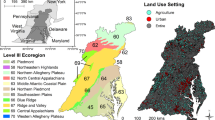
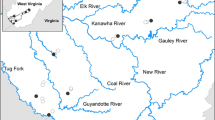
Mayflies (Order Ephemeroptera) require high quality water and habitat in streams to thrive, so their appearance after restoration is an indicator of ecological recovery. To better understand the importance of restoring in-stream habitat versus water chemistry for macroinvertebrate communities, we developed taxon-specific models of occurrence for five mayfly genera (Caenis, Isonychia, Stenonema, Stenacron, and Baetis) inhabiting streams in the Appalachian Mountains, USA. Presence/absence records from past decades were used to develop single and multiple logistic predictive models based on catchment characteristics (drainage area, gradient), in-stream habitat variables (e.g., substrate, channel morphology, pool and riffle quality), and water chemistry. Model performance was evaluated using (a) classification rates and Hosmer-Lemeshow values for test sets of data withheld from the original model-building dataset and (b) a field comparison of predicted versus observed mayfly occurrences at 53 sites in acid mine drainage-impaired watersheds in 2012. The classification accuracies of final models for Caenis, Stenacron, and Baetis ranged from 50 to 75%. In-stream habitat features were not significant predictor variables for these three taxa, only water chemistry. Models for Isonychia and Stenonema had higher classification rates (81%) and included both habitat and chemical variables. However, actual occurrences of Isonychia and Stenonema at study sites in 2012 were low, consistent with the calculated probability of occurrence (Po) < 0.60. Caenis occurred at test sites 35% of the time when the model predicted a Po > 0.40. Stenacron showed the greatest consistency of actual versus predicted occurrences, occurring at 56% of sites when the Po (based on pH and conductivity) was > 0.50 and only at 1 site when Po < 0.5. The results demonstrate how predictive models of individual indicator taxa could be valuable for evaluating the relative impacts of restoring physical habitat versus water chemistry during stream remediation.



Similar content being viewed by others
References
Ahmadi-Nedushan, B., St‐Hilaire, A., Bérubé, M., Robichaud, É., Thiémonge, N., & Bobée, B. (2006). A review of statistical methods for the evaluation of aquatic habitat suitability for instream flow assessment. River Research and Applications, 22(5), 503–523.
Battaglia, M., Hose, G. C., Turak, E., & Warden, B. (2005). Depauperate macroinvertebrates in a mine affected stream: clean water may be the key to recovery. Environmental Pollution, 138(1), 132–141.
Beechie, T. J., & Sibley, T. H. (1997). Relationships between channel characteristics, woody debris, and fish habitat in northwestern Washington streams. Transactions of the American Fisheries Society, 126(2), 217–229.
Bott, T. L., Jackson, J. K., McTammany, M. E., Newbold, J. D., Rier, S. T., Sweeney, B. W., & Battle, J. M. (2012). Abandoned coal mine drainage and its remediation: impacts on stream ecosystem structure and function. Ecological Applications, 22(8), 2144–2163.
Bowman, J. and Johnson, K. S. (2016) 2015 comprehensive non-point source (NPS) monitoring report for acid mine drainage (AMD), http://www.watersheddata.com/UserView_Report.aspx
Burnham, K. P., & Anderson, D. R. (2002). Model selection and multimodel inference (2nd ed.). New York: Springer.
Carlisle, D. M., & Clements, W. H. (2005). Leaf litter breakdown, microbial respiration and shredder production in metal-polluted streams. Freshwater Biology, 50(2), 380–390.
Churchel, M. A., & Batzer, D. P. (2006). Recovery of aquatic macroinvertebrate communities from drought in Georgia Piedmont headwater streams. The American Midland Naturalist, 156(2), 259–272.
Cohen, R. R. H., & Gorman, J. (1991). Mining-related nonpoint source pollution. Water Environment and Technology, 3, 55–59.
Corkum, L. D. (1989). Habitat characterization of the morphologically similar mayfly larvae, Caenis and Tricorythodes (Ephemeroptera). Hydrobiologia, 179(2), 103–109.
Cormier, S. M., Suter, G. W., & Zheng, L. (2013). Derivation of a benchmark for freshwater ionic strength. Environmental Toxicology and Chemistry, 32(2), 263–271.
Cruz, M. J., Rebelo, R., & Crespo, E. (2006). Effects of an introduced crayfish, Procambarus clarkii, on the distribution of south-western Iberian amphibians in their breeding habitats. Ecography, 29(3), 329–338.
Cruz, M. J., & Rebelo, R. (2007). Colonization of freshwater habitats by an introduced crayfish, Procambarus clarkii, in Southwest Iberian Peninsula. Hydrobiologia, 575(1), 191–201.
Edwards, R. T., & Meyer, J. L. (1990). Bacterivory by deposit-feeding mayfly larvae (Stenonema spp.) Freshwater Biology, 24(3), 453–462.
Eyre, M. D., Pilkington, J. G., Carr, R., McBlane, R. P., Rushton, S. P., & Foster, G. N. (1993). The running-water beetles (Coleoptera) of a river catchment in northern England. Hydrobiologia, 264(1), 33–45.
Echols, B. S., Currie, R. J., & Cherry, D. S. (2010). Preliminary results of laboratory toxicity tests with the mayfly, Isonychia bicolor (Ephemeroptera: Isonychiidae) for development as a standard test organism for evaluating streams in the Appalachian coalfields of Virginia and West Virginia. Environmental Monitoring and Assessment, 169(1–4), 487–500.
Ferrier, S., Drielsma, M., Manion, G., & Watson, G. (2002). Extending statistical approaches to modelling spatial patternin biodiversity in northeast New South Wales. II. Community-level modelling. Biodiversity and Conservation, 11, 2309–2338.
Fritz, K. M., & Dodds, W. K. (2004). Resistance and resilience of macroinvertebrate assemblages to drying and flood in a tallgrass prairie stream system. Hydrobiologia, 527(1), 99–112.
Fuchs, U., & Statzner, B. (1990). Time scales for the recovery potential of river communities after restoration: lessons to be learned from smaller streams. River Research and Applications, 5(1), 77–87.
Gjerløv, C., Hildrew, A. G., & Jones, J. I. (2003). Mobility of stream invertebrates in relation to disturbance and refugia: a test of habitat templet theory. Journal of the North American Benthological Society, 22(2), 207–223.
Gore, J. A., Layzer, J. B., & Mead, J. (2001). Macroinvertebrate in stream flow studies after 20 years: a role in stream management and restoration. Regulated Rivers: Research & Management, 17, 527–542.
Gore, J. A., & Bryant Jr., R. M. (1990). Temporal shifts in physical habitat of the crayfish, Orconectes neglectus (Faxon). Hydrobiologia, 199(2), 131–142.
Gray, N. F. (1997). Environmental impact and remediation of acid mine drainage: a management problem. Environmental Geology, 30(1–2), 62–71.
Harries, J. R. (1997). Acid mine drainage in Australia: its extent and potential future liability. Canberra: Supervising Scientist.
Hawkins, C. P., Norris, R. H., Hogue, J. N., & Feminella, J. W. (2000). Development and evaluation of predictive models for measuring the biological integrity of streams. Ecological Applications, 10, 1456–1477.
Hamsher, S. E., Verb, R. G., & Vis, M. L. (2004). Analysis of acid mine drainage impacted streams using a periphyton index. Journal of Freshwater Ecology, 19(2), 313–324.
Hastie, L. C., Cooksley, S. L., Scougall, F., Young, M. R., Boon, P. J., & Gaywood, M. J. (2003). Characterization of freshwater pearl mussel (Margaritifera Mmargaritifera) riverine habitat using River Habitat Survey data. Aquatic Conservation: Marine and Freshwater Ecosystems, 13(3), 213–224.
Heino, J., & Mykrä, H. (2006). Assessing physical surrogates for biodiversity: do tributary and stream type classifications reflect macroinvertebrate assemblage diversity in running waters? Biological Conservation, 129(3), 418–426.
Hogsden, K. L., & Harding, J. S. (2011). Consequences of acid mine drainage for the structure and function of benthic stream communities: a review. Freshwater Science, 31(1), 108–120.
Hogsden, K. L., & Harding, J. S. (2012). Anthropogenic and natural sources of acidity and metals and their influence on the structure of stream food webs. Environmental Pollution, 162, 466–474.
Holland-Bartels, L. E. (1990). Physical factors and their influence on the mussel fauna of a main channel border habitat of the upper Mississippi River. Journal of the North American Benthological Society, 9(4), 327–335.
Hosmer, D. W., & Lemeshow, S. (1989). Applied regression analysis. New York: John Wiley.
IBM Corp. (2013). IBM SPSS Statistics for Windows, version 22.0. Armonk: IBM Corp.
Jennings, S. R., Blicker, P. S., & Neuman, D. R. (2008). Acid mine drainage and effects on fish health and ecology: a review. Bozeman: Reclamation Research Group.
Johnson, K. S. (2007). Field and laboratory methods for using the MAIS (Macroinvertebrate Aggregated Index for Streams) in Rapid Bioassessment of Ohio Streams. Can be accessed at http://www.epa.ohio.gov/dsw/credibledata/references.aspx. Accessed 28 Feb 2018
Johnson, D. B., & Hallberg, K. B. (2005). Acid mine drainage remediation options: a review. Science of the Total Environment, 338(1), 3–14.
Johnson, K. S., Thompson, P. C., Gromen, L., & Bowman, J. (2014). Spatial patterns in macroinvertebrate recovery and leaf litter breakdown in an acid mine impacted stream treated with an active alkaline doser. Environmental Monitoring and Assessment, 186(7), 4111–4127.
Joy, M. K., & Death, R. G. (2004). Predictive modelling and spatial mapping of freshwater fish and decapod assemblages using GIS and neural networks. Freshwater Biology, 49(8), 1036–1052.
Karr, J. R. (1995). Protecting aquatic ecosystems: clean water is not enough. WS Davis and TP Simon. Biological assessment and criteria. Tools for water resource planning and decision making (pp. 7–13). Boca Raton: Lewis Publishers.
Kennedy, A. J., Cherrry, D. S., & Currie, R. J. (2004). Evaluation of ecologically relevant bioassays for a lotic system impacted by a coal-mine effluent, using Isonychia. Environmental Monitoring and Assessment, 95(1–3), 37–55.
Knapp, R. A., & Preisler, H. K. (1999). Is it possible to predict habitat use by spawning salmonids? A test using California golden trout (Oncorhynchus mykiss aguabonita). Canadian Journal of Fisheries and Aquatic Sciences, 56(9), 1576–1584.
Kondratieff, B. C., & Voshell Jr., J. R. (1984). The North and Central American species of Isonychia (Ephemeroptera: Oligoneuriidae). Transactions of the American Entomological Society, 1, 129–244.
Kowalik, R. A., Cooper, D. M., Evans, C. D., & Ormerod, S. J. (2007). Acidic episodes retard the biological recovery of upland British streams from chronic acidification. Global Change Biology, 13(11), 1365–2486.
Kruse, N. A., Bowman, J. R., Mackey, A. L., McCament, B., & Johnson, K. S. (2012). The lasting impacts of offline periods in lime dosed streams: a case study in Raccoon Creek, Ohio. Mine Water and the Environment, 31(4), 266–272.
Leftwich, K. N., Angermeier, P. L., & Dolloff, C. A. (1997). Factors influencing behavior and transferability of habitat models for a benthic stream fish. Transactions of the American Fisheries Society, 126(5), 725–734.
Letovsky, E., Myers, I. E., Canepa, A., & McCabe, D. J. (2012). Differences between kick sampling techniques and short-term Hester-Dendy sampling for stream macroinvertebrates. Bios, 83(2), 47–55.
Light, T. (2003). Success and failure in a lotic crayfish invasion: the roles of hydrologic variability and habitat alteration. Freshwater Biology, 48(10), 1886–1897.
Linke, S., Norris, R. H., Faith, D. P., & Stockwell, D. (2005). ANNA: a new prediction method for bioassessment programs. Freshwater Biology, 50(1), 147–158.
Louhi, P., Mykrä, H., Paavola, R., Huusko, A., Vehanen, T., Mäki-Petäys, A., & Muotka, T. (2011). Twenty years of stream restoration in Finland: little response by benthic macroinvertebrate communities. Ecological Applications, 21(6), 1950–1961.
McCabe, D. J., Hayes-Pontius, E. M., Canepa, A., Berry, K. S., & Levine, B. C. (2012). Measuring standardized effect size improves interpretation of biomonitoring studies and facilitates meta-analysis. Freshwater Science, 31(3), 800–812.
Macanowicz, N., Boeing, W. J., & Gould, W. R. (2013). Evaluation of methods to assess benthic biodiversity of desert sinkholes. Freshwater Science, 32(4), 1101–1110.
Manel, S., Dias, J., & Ormerod, S. J. (1999). Comparing discriminant analysis, neural networks and logistic regression for predicting species distributions: a case study with a Himalayan river bird. Ecological Modelling, 120(2), 337–347.
Manel, S., Williams, H. C., & Ormerod, S. J. (2001). Evaluating presence–absence models in ecology: the need to account for prevalence. Journal of Applied Ecology, 38, 921–931.
Meier, P. G., Penrose, D. L., & Polak, L. (1979). The rate of colonization by macro-invertebrates on artificial substrate samplers.
Merritt, R. W., & Cummins, K. W. (1996). An introduction to the aquatic insects of North America. Dubuque: Kendall Hunt.
Miller, S. W., Budy, P., & Schmidt, J. C. (2009). Quantifying macroinvertebrate responses to instream habitat restoration: applications of meta-analyses to river restoration. Restoration Ecology, 18, 8–19.
Modde, T., & Drewes, H. G. (1990). Comparison of biotic index values for invertebrate collections from natural and artificial substrates. Freshwater Biology, 23(2), 171–180.
Murphy, J. F., Winterbottom, J. H., Orton, S., Simpson, G. L., Shilland, E. M., & Hildrew, A. G. (2014). Evidence of recovery from acidification in the macroinvertebrate assemblages of UK fresh waters: a 20-year time series. Ecological Indicators, 37, 330–340.
Naidoo, B. (2009). Acid mine drainage single most significant threat to South Africa’s environment. Mining Weekly, 8.
Nelson, S. M., & Roline, R. A. (1996). Recovery of a stream macroinvertebrate community from mine drainage disturbance. Hydrobiologia, 339(1–3), 73–84.
Niyogi, D. K., Lewis Jr., W. M., & McKnight, D. M. (2001). Litter breakdown in mountain streams affected by mine drainage: biotic mediation of abiotic controls. Ecological Applications, 11(2), 506–516.
Niyogi, D. K., Harding, J. S., & Simon, K. S. (2013). Organic matter breakdown as a measure of stream health in New Zealand streams affected by acid mine drainage. Ecological Indicators, 24, 510–517.
O'Halloran, K., Cavanagh, J. A., & Harding, J. S. (2008). Response of a New Zealand mayfly (Deleatidium spp.) to acid mine drainage: implications for mine remediation. Environmental Toxicology and Chemistry, 27(5), 1135–1140.
Ohio, E. P. A. (1989). Biological criteria for the protection of aquatic life. In Standardized biological field sampling and laboratory methods for assessing fish and macroinvertebrate communities (Vol. III). Columbus: Ohio Environmental Protection Agency.
Ohio, E. P. A. (2006). Methods for assessing habitat in flowing waters: using the qualitative habitat evaluation index (QHEI). Technical Bulletin EAS/2006-06-01.
Ohio, E. P. A. (1999). Ohio EPA five year monitoring surface water monitoring and assessment strategy, 2000–2004. Ohio EPA Tech. Bu ll. MAS/1999–7-2. Ohio: Division of Surface Water, Monitoring and Assessment Section, Columbus.
Olden, J. D. (2003). A species-specific approach to modeling biological communities and its potential for conservation. Conservation Biology, 17, 854–863.
Olden, J. D., Joy, M. K., & Death, R. G. (2006). Rediscovering the species in community-wide predictive modeling. Ecological Applications, 16(4), 1449–1460.
Omernik, J. M. (1987). Ecoregions of the conterminous United States. Annals of the Association of American Geographers, 77(1), 118–125.
Palmer, M. A., Menninger, H., & Bernhardt, E. (2010). River restoration, habitat heterogeneity and biodiversity: a failure of theory or practice? Freshwater Biology, 55(Supplement 1), 205–222.
Pander, J., & Geist, J. (2013). Ecological indicators for stream restoration success. Ecological Indicators, 30, 106–118.
Park, Y. S., Verdonschot, P. F., Chon, T. S., & Lek, S. (2003). Patterning and predicting aquatic macroinvertebrate diversities using artificial neural network. Water research, 37(8), 1749–1758.
Peeters, E. T. (1998). Logistic regression as a tool for defining habitat requirements of two common gammarids. Freshwater Biology, 39(4), 605–615.
Pond, G. J. (2010). Patterns of Ephemeroptera taxa loss in Appalachian headwater streams (Kentucky, USA). Hydrobiologia, 641(1), 185–201.
Pond, G. J. (2012). Biodiversity loss in Appalachian headwater streams (Kentucky, USA): Plecoptera and Trichoptera communities. Hydrobiologia, 679(1), 97–117.
Pond, G. J., Passmore, M. E., Pointon, N. D., Felbinger, J. K., Walker, C. A., Krock, K. J., Fulton, J. B., & Nash, W. L. (2014). Long-term impacts on macroinvertebrates downstream of reclaimed mountaintop mining valley fills in central Appalachia. Environmental Management, 54(4), 919–933.
Puckett, R. T., & Cook, J. L. (2004). Physiological tolerance ranges of larval Caenis latipennis (Ephemeroptera: Caenidae) in response to fluctuations in dissolved oxygen concentration, pH and temperature. Texas Journal of Science, 56(2), 123–130.
Rader, R. B. (1997). A functional classification of the drift: traits that influence invertebrate availability to salmonids. Canadian Journal of Fisheries and Aquatic Sciences, 54(6), 1211–1234.
Ramcharan, C. W., Padilla, D. K., & Dodson, S. I. (1992). Models to predict potential occurrence and density of the zebra mussel, Dreissena polymorpha. Canadian Journal of Fisheries and Aquatic Sciences, 49(12), 2611–2620.
Rankin, E. T. (1989). The qualitative habitat evaluation index (QHEI): rationale, methods, and application. Div. Water Qual. Plan. & Assess., Ecol. Assess. Sect., Columbus.
Rankin, E. T. (1995). Habitat indices in water resource quality assessment. In W. S. Davis & T. P. Simon (Eds.), Biological assessment and criteria (pp. 181–214). Boca Raton: Lewis Publishers.
Randolph, R. P., & McCafferty, W. P. (1998). Diversity and distribution of the mayflies (Ephemeroptera) of Illinois, Indiana, Kentucky, Michigan, Ohio and Wisconsin. Ohio Biological Survey Bulletin New Series, 13(1), 1–188.
Richards, C., & Minshall, G. W. (1992). Spatial and temporal trends in stream macroinvertebrate communities: the influence of catchment disturbance. Hydrobiologia, 241(3), 173–194.
Rinella, D. J., & Feminella, J. W. (2005). Comparison of benthic macroinvertebrates colonizing sand, wood, and artificial substrates in a low-gradient stream. Journal of Freshwater Ecology, 20(2), 209–220.
Rosenberg, D. M., & Resh, V. H. (1982). The use of artificial substrates in the study of freshwater benthic macroinvertebrates. In J. Cairns (Ed.), Artificial substrates (pp. 175–235). Ann Arbor: Ann Arbor Science.
Simmons, J. A., Lawrence, E. R., & Jones, T. G. (2005). Treated and untreated acid mine drainage effects on stream periphyton biomass, leaf decomposition, and macroinvertebrate diversity. Journal of Freshwater Ecology, 20(3), 413–424.
Smith, E. P., & Voshell, J. R. (1997). Studies of benthic macroinvertebrates and fish in streams within EPA region 3 for development of biological indicators of ecological condition. Blacksburg: Virginia Polytechnic Institute and State University.
Snucins, E. (2003). Recolonization of acid-damaged lakes by the benthic invertebrates Stenacron interpunctatum, Stenonema femoratum and Hyalella azteca. Ambio: A Journal of the Human Environment, 32(3), 225–229.
Solà, C., Burgos, M., Plazuelo, Á., Toja, J., Plans, M., & Prat, N. (2004). Heavy metal bioaccumulation and macroinvertebrate community changes in a Mediterranean stream affected by acid mine drainage and an accidental spill (Guadiamar River, SW Spain). Science of the Total Environment, 333(1), 109–126.
Steyerburg, E. (2009). Clinical prediction models: a practical approach to development, validation and updating, (Chapters 9 & 12). New York: Springer-Verlag.
Symonds, M. R., & Moussalli, A. (2011). A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behavioral Ecology and Sociobiology, 65(1), 13–21.
Taylor, J. M., & Kennedy, J. H. (2006). Life history and secondary production of Caenis latipennis (Ephemeroptera: Caenidae) in Honey Creek, Oklahoma. Annals of the Entomological Society of America, 99(5), 821–830.
Tronstad, L. M., & Hotaling, S. (2017). Long-term trends in aquatic ecosystem bioassessment metrics are not influenced by sampling method: empirical evidence from the Niobrara River. Knowledge and Management of Aquatic Ecosystems, (418), 28.
U.S. Environmental Protection Agency. (2002). Mid-Atlantic acidification, report, Washington, D. C. Available at http://www.epa.gov/region03/acidification.
Usio, N. (2007). Endangered crayfish in northern Japan: distribution, abundance and microhabitat specificity in relation to stream and riparian environment. Biological Conservation, 134(4), 517–526.
U.S. Department of the Interior | U.S. Geological Survey URL: http://streamstats.usgs.gov/ Page Contact Information: GS-W_Streamstats@usgs.gov Page Last Modified: Monday, 13-Jul-2015 09:44:12 EDT.
Van Sickle, J., & Hughes, R. M. (2000). Classification strengths of ecoregions, catchments, and geographic clusters for aquatic vertebrates in Oregon. Journal of the North American Benthological Society, 19(3), 370–384.
Verb, R. G., & Vis, M. L. (2005). Periphyton assemblages as bioindicators of mine-drainage in unglaciated Western Allegheny Plateau lotic systems. Water, Air, and Soil Pollution, 161(1–4), 227–265.
Walter, C. A., Nelson, D., & Earle, J. I. (2012). Assessment of stream restoration: sources of variation in macroinvertebrate recovery throughout an 11-year study of coal mine drainage treatment. Restoration Ecology, 20(4), 431–440.
Acknowledgements
We thank the American Electric Power Foundation and the Ohio Department of Natural Resources, Division of Mineral Resources for funding and the Environmental Studies program at Ohio University and Voinovich School for general support for students, faculty, and staff. Steve Porter and Liz Migliore assisted with statistical analyses and graphics. Jeff Calhoun of Ohio Department of Natural Resources helped conduct habitat assessments for the study sites and we especially thank Michele Shively, Amy Mackey, Sarah Landers, and Nate Schlater for invaluable assistance with water chemistry and biological collection. Special thanks go to Pete Thompson for help with mayfly enumeration and identification.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary Table 1
(DOCX 21 kb).
Rights and permissions
About this article
Cite this article
Johnson, K.S., Rankin, E., Bowman, J. et al. Predicting mayfly recovery in acid mine-impaired streams using logistic regression models of in-stream habitat and water chemistry. Environ Monit Assess 190, 196 (2018). https://doi.org/10.1007/s10661-018-6548-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6548-z