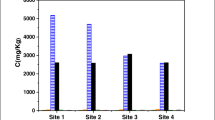
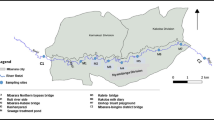
Abstract
Speciation is defined as the distribution of an element among different chemical species. Although the relation between speciation and bioavailability is complex, the metal present as free hydrated ion, or as weak complexes able to dissociate, is usually more bioavailable than the metal incorporated in strong complexes or adsorbed on colloidal or particulate matter. Among the analytical techniques currently available, anodic stripping voltammetry (ASV) has been one of the most used in the identification and quantification of several heavy metal species in aquatic systems. This work concerns the speciation study of lead, in original (natural, non-filtered) and filtered water samples and in suspensions of particulate matter and sediments from Luanda Bay (Angola). Complexes of lead with organics were identified and quantified by differential pulse anodic stripping voltammetry technique. Each sample was progressively titrated with a Pb(II) standard solution until complete saturation of the organic ligands. After each addition of Pb(II), the intensity, potential and peak width of the voltammetric signal were measured. The results obtained in this work show that more than 95 % of the lead in the aquatic environment is bound in inert organic complexes, considering all samples from different sampling sites. In sediment samples, the lead is totally (100 %) complexed with ligands adsorbed on the particles surface. Two kinds of dominant lead complexes, very strong (logK >11) and strong to moderately strong (8< logK <11), were found, revealing the lead affinity for the stronger ligands.





Similar content being viewed by others
References
Bi, Z., Salaün, P., & Van der Berg, C. (2013). The speciation of lead in seawater by pseudopolarography using a vibrating silver amalgam microwave electrode. Marine Chemistry, 151, 1–12.
Bonnail, E., Sarmiento, A., Delvalls, T., Nieto, J., & Riba, I. (2016). Assessment of metal contamination, bioavailability, toxicity and bioaccumulation in extreme metallic environments (Iberian Pyrite Belt) using Corbicula fluminea. Science of the Total Environment, 544, 1031–1044.
Botelho, C. (1998). Especiação de iões metálicos em três estações do Rio Este em diferentes épocas do ano. Ph.D. Thesis, Faculty of Engineering, University of Porto, Portugal.
Bruland, W., Rue, E., Donat, J., Skrabal, S., & Moffett, J. (2000). Intercomparison of voltammetric techniques to determine the chemical speciation of dissolved copper in a coastal seawater sample. Analytica Chimica Acta, 405, 99–113.
Ciffroy, P., Garnier, J.-M., & Benyahya, L. (2003). Kinetic partitioning of Co, Mn, Cs, Fe, Ag, Zn and Cd in fresh waters (Loire) mixed with brackish waters (Loire estuary): experimental and modelling approaches. Marine Pollution Bulletin, 46, 626–641.
Cobelo-Garcia, A., & Prego, R. (2004). Chemical speciation of dissolved copper, lead and zinc in a ria coastal system: the role of resuspended sediments. Analytica Chimica Acta, 524, 109–114.
De Pippo, T., Duarte Morais, M. (1994). Man induced morphological and sedimentary changes on ilha de Luanda and ilha de Chicala (Angola). In Littoral 94 (pp. 107–117), September 26–29, Lisbon, Portugal.
DeFord, D., & Hume, D. (1951). The determination of consecutive formation constants of complex ions from polarographic data. Journal of the American Chemical Society, 73(11), 5321–5322.
Florence, T., Lumsden, B., & Fardy, J. (1983). Evaluation of some physico-chemical techniques for the determination of the fraction of dissolved copper toxic to the marine diatom Nitzchia closterium. Analytica Chimica Acta, 15, 281–295.
Guéguen, C., Gilbin, R., Pardos, M., & Dominik, J. (2004). Water toxicity and metal contamination assessment of a polluted river: the upper Vistula River (Poland. Applied Geochemistry, 19, 153–162.
Hoffmann, S. (2002). Strong binding of copper, zinc, and lead to colloids and natural matter in rivers. Dissertation submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy, Wisconsin-Madison University.
Hudson, R. (1998). Which aqueous species control the rates of trace metal uptake by aquatic biota? Observations and predictions of non-equilibrium effects. The Science of the Total Environment, 219, 95–115.
Kotas, J., & Stasicka, Z. (2000). Chromium occurrence in the environment and methods for its speciation. Environmental Pollution, 107, 263–283.
Kozelka, P., & Bruland, K. (1998). Chemical speciation of dissolved Cu, Zn, Cd, Pb in Narragansett Bay, Rhode Island. Marine Chemistry, 60, 267–282.
Kozelka, P., Sañudo-Wilhelmy, S., Flegal, A., & Bruland, K. (1997). Physico-chemical speciation of lead in South San Francisco Bay. Estuarine, Coastal and Shelf Science, 44, 649–658.
Leleyter, L., & Baraud, F. (2005). Évaluation de la mobilité des métaux dans les sédiments fluviaux du bassin de la Vire (Normandie, France) par extractions simples ou séquentielles. Comptes Rendus Geoscience, 337, 571–579.
Li, L., Liu, J., Wang, X., & Shi, X. (2015). Dissolved trace metal distributions and Cu speciation in the southern Bohai Sea, China. Marine Chemistry, 172, 34–45.
LNEC (1980). Esquema do sistema lagunar em Luanda: Construção e taragem do modelo. Laboratório Nacional de Engenharia Civil. Estudo realizado a pedido do antigo Ministério do Ultramar- Direcção-Geral de Obras Públicas e Comunicações. Relatório da 1ª Fase, pag.3.
Magnier, A., Billon, G., Louis, Y., Baeyens, W., & Elskens, M. (2011). On the lability of dissolved Cu, Pb and Zn in freshwater: optimization and application to the Deûle (France. Talanta, 86, 91–98.
Markich, S., Brown, P., Batley, G., Apte, S., & Stauber, J. (2001). Incorporation of metal speciation and bioavailability into water quality guidelines for protecting aquatic ecosystems. Australasian Journal of Ecotoxicology, 7, 109–122.
Meers, E., Unamuno, V., Du Laing, G., Vangronsveld, J., Vanbroekhoven, K., Samson, R., Diels, L., Geebelen, W., Ruttens, A., Vandegehuchte, M., & Tack, F. (2006). Zn in the soil solution of unpolluted and polluted soils as affected by soil characteristics. Geoderma, 136, 107–119.
Mester, Z., Cremisini, C., Ghiara, E., & Morabito, R. (1998). Comparison of two sequential extraction procedures for metal fractionation in sediment samples. Analytica Chimica Acta, 359, 133–142.
Mota, A., Cruz, P., Vilhena, C., & Gonçalves, M. (2005). Influence of the sediment on lead speciation in the Tagus estuary. Water Research, 39, 1451–1460.
Muller, F. (1996). Interactions of copper, lead, and cadmium with the dissolved, colloidal and particulate components of estuarine and coastal waters. Marine Chemistry, 52, 245–268.
Ruzic, I. (1982). Theoretical aspects of the direct titration of natural waters and its information yield for trace metal speciation. Analytica Chimica Acta, 140, 99–113.
Ruzic, I. (1996). Trace metal complexation at heterogeneous binding sites in aquatic systems. Marine Chemistry, 53, 1.
Salam, M. (2003). Determination of Cd(II), Cu (II), Zn (II), and Pb (II) speciation in aqueous solutions by electrochemical techniques. Thesis submitted in partial fulfilment of the requirements for the degree of Master in Science, Carleton University.
Salomons, W. (1995). Environmental impact of metals derived from mining activities: processes, predictions, prevention. Journal Geochemical Exploration, 52, 5–23.
Santos, A. (2012). Estudo da Qualidade da Água da Baía de Luanda: Distribuição de Metais Pesados na Água, Sólidos Suspensos e Sedimentos. Ph.D. Thesis, Faculty of Engineering, Agostinho Neto University, Angola.
Santos, A., Leitão, A., & Boaventura, R. (2011). Heavy metal pollution of sediments from coastal areas: assessment of the environmental impact of polluted surface sediments fom the bay of Luanda, Angola. Journal of Environmental Science and Engineering, 5, 1317–1336.
Scatchard, G. (1949). The attractions of proteins for small molecules and ions. Analytical New York Academy. Science, 51, 660–672.
Sharma, V., Chaudhary, P., & Satyanarayan, S. (2011). Toxicity assessment of free form of heavy metals in aqueous media on earthworm Eudrillus eugeniae. Water Science and Technology, 2011, 2434–2445.
Tang, D., Warnken, K., & Santschi, P. (2002). Distribution and partitioning of trace metals (Cd, Cu, Ni, Pb, Zn) in Galveston Bay waters. Marine Chemistry, 78, 29–45.
Tonietto, A., Lombardi, A., Choueri, R., & Vieira, A. (2015). Chemical behaviour of Cu, Zn, Cd and Pb in a eutrophic reservoir: speciation and complexation capacity. Environmental Science Pollution Research, 22, 15920–15930.
Town, R., & Filella, M. (2002). Implications of natural organic matter binding heterogeneity on understanding lead (II) complexation in aquatic systems. The Science of the Total Environment, 300, 143–154.
Twiss, M., Errécalde, O., Fortin, C., Campbell, P., Jumarie, C., Denizeau, F., Berkelaar, E., Hale, B., & Van Rees, K. (2001). Coupling the use of computer chemical speciation models and culture techniques in laboratory investigations of trace metal toxicity. Chemical Speciation and Bioavailability, 13, 9.
Van den Berg, C., & Kramer, J. (1984). Determination of complexing capacity and conditional stability constants of complexes of copper (II) with natural organic ligands in seawater by cathodic stripping voltammetry of copper-catechol complex ions. Marine Chemistry, 15, 1–18.
Vasconcelos, M., & Leal, M. (1997). Speciation of Cu, Pb, Cd and Hg in waters of the Oporto coast in Portugal using pre-concentration in a chelamine resin column. Analytica Chimica Acta, 353, 189–198.
Waeles, M., Maguer, J.-F., & Le Corre, P. (2004). Distribution and chemical speciation of dissolved cadmium and copper in the Loire estuary and North Biscay continental shelf France. Estuarine, Coastal and Shelf Science, 59, 49–57.
Wells, M., Kozelka, P., & Bruland, K. (1998). The complexation of “dissolved” Cu, Zn, Cd e Pb by soluble and colloidal organic matter in Narragansett Bay. Marine Chemistry, 62, 203–217.
Acknowledgments
Ana Maria Santos acknowledges the Calouste Gulbenkian Foundation (Portugal) for the Ph.D. scholarship and the SONILS and port of Luanda for the support in the sampling operations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leitão, A., Santos, A.M. & Boaventura, R.A.R. Complexation of lead by organic matter in Luanda Bay, Angola. Environ Monit Assess 188, 563 (2016). https://doi.org/10.1007/s10661-016-5557-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5557-z