Abstract
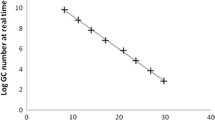
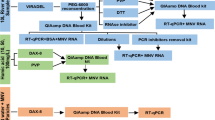
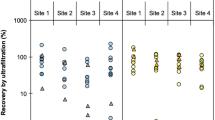
Enteric viruses monitoring in surface waters requires the concentration of viruses before detection assays. The aim of this study was to evaluate different methods in terms of recovery efficiencies of bacteriophage PP7 of Pseudomonas aeruginosa, measured by real-time PCR, using it as a viral control process in water analysis. Different nucleic acid extraction methods (silica–guanidinium thiocyanate, a commercial kit (Qiagen Viral RNA Kit) and phenol–chloroform with alcohol precipitation) exhibited very low recovery efficiencies (0.08–4.18 %), being the most efficient the commercial kit used for subsequent experiments. To evaluate the efficiency of three concentration methods, PBS (as model for clean water) and water samples from rivers were seeded to reach high (HC, 106 pfu ml−1) and low concentrations (LC, 104 pfu ml−1) of PP7. Tangential ultrafiltration proved to be more efficient (50.36 ± 12.91, 17.21 ± 9.22 and 12.58 ± 2.35 % for HC in PBS and two river samples, respectively) than adsorption–elution with negatively charged membranes (1.00 ± 1.34, 2.79 ± 2.62 and 0.05 ± 0.08 % for HC in PBS and two river samples, respectively) and polyethylene glycol precipitation (15.95 ± 7.43, 4.01 ± 1.12 and 3.91 ± 0.54 %, for HC in PBS and two river samples, respectively), being 3.2–50.4 times more efficient than the others for PBS and 2.7–252 times for river samples. Efficiencies also depended on the initial virus concentration and aqueous matrixes composition. In consequence, the incorporation of an internal standard like PP7 along the process is useful as a control of the water concentration procedure, the nucleic acid extraction, the presence of inhibitors and the variability of the recovery among replicas, and for the calculation of the sample limit of detection. Thus, the use of a process control, as presented here, is crucial for the accurate quantification of viral contamination.
Similar content being viewed by others
References
Albinana-Gimenez, N., Clemente-Casares, P., Calgua, B., Huguet, J. M., Courtois, S., & Girones, R. (2009). Comparison of methods for concentrating human adenoviruses, polyomavirus JC, and noroviruses in source waters and drinking water using quantitative PCR. Journal of Virological Methods, 158, 104–109.
Aranha-Creado, H., & Brandwein, H. (1999). Application of bacteriophages as surrogates for mammalian viruses: a case for use in filter validation based on precedents and current practices in medical and environmental virology. Journal of Pharmaceutical Science and Technology, 53(2), 75–82.
Atha, D. H., & Ingham, K. C. (1981). Mechanism of precipitation of proteins by polyethylene glycols: analysis in terms of excluded volumes. The Journal of Biological Chemistry, 256, 12108–12117.
Bae, J., & Schwab, K. J. (2008). Evaluation of murine norovirus, feline calicivirus, poliovirus, and MS2 as surrogates for human norovirus in a model of viral persistence in surface water and groundwater. Applied and Environmental Microbiology, 74(2), 477–484.
Blatchley, E. R., Gong, W. L., Alleman, J. E., Rose, J. B., Huffman, D. E., Otaki, M., & Lisle, J. T. (2007). Effects of wastewater disinfection on waterborne bacteria and viruses. Water Environmental Research, 79(1), 81–92.
Boom, R., Sol, C. J., Salimans, M. M., Jansen, C. L., Wertheim-van Dillen, P. M., & van der Noordaa, J. (1990). Rapid and simple method for purification of nucleic acids. Journal of Clinical Microbiology, 28(3), 495–503.
Costafreda, M. I., Bosch, A., & Pinto, R. M. (2006). Development, evaluation, and standardization of a real-time TaqMan reverse transcription-PCR assay for quantification of hepatitis A virus in clinical and shellfish samples. Applied and Environmental Microbiology, 72(6), 3846–3855.
D’Souza, D. H., & Su, X. (2010). Efficacy of chemical treatments against murine norovirus, feline calicivirus, and MS2 bacteriophage. Foodborne Pathogens and Disease, 7(3), 319–326.
Espinosa, A. C., Mazari-Hiriart, M., Espinosa, R., Maruri-Avidal, L., Méndez, E., & Arias, C. F. (2008). Infectivity and genome persistence of rotavirus and astrovirus in groundwater and surface water. Water Research, 42(10–11), 2618–2628.
Farrah, S. R. (1982). Chemical factors influencing adsorption of bacteriophage MS2 to membrane filters. Applied and Environmental Microbiology, 43(3), 659–663.
Ferguson, C. M., Coote, B. G., Ashbolt, N. J., & Stevenson, I. M. (1996). Relationships between indicators, pathogens and water quality in an estuarine system. Water Research, 30, 2045–2054.
Greening, G. E., Hewitt, J., & Lewis, G. D. (2002). Evaluation of integrated cell 3 culture-PCR (C-PCR) for virological analysis of environmental samples. Journal of Applied Microbiology, 93, 745–750.
Horm, K. M., & D’Souza, D. H. (2011). Survival of human norovirus surrogates in milk, orange, and pomegranate juice, and juice blends at refrigeration (4 °C). Food Microbiology, 28, 1054–1061.
Huang, Q. S., Greening, G., Baker, M. G., Grimwood, K., Hewitt, J., Hulston, D., van Duin, L., Fitzsimons, A., Garrett, N., Graham, D., Lennon, D., Shimizu, H., Miyamura, T., & Pallansch, M. A. (2005). Persistence of oral polio vaccine virus after its removal from the immunization schedule in New Zealand. Lancet, 366, 394–396.
Katayama, H., Shimazaki, A., & Ohgaki, S. (2002). Development of a virus concentration method and its application to detection of enterovirus and norwalk virus from coastal seawater. Applied and Environmental Microbiology, 68(3), 1033–1039.
Lee, J. C., & Lee, L. L. Y. (1981). Preferential solvent interactions between proteins and polyethylene glycols. The Journal of Biological Chemistry, 256(2), 625–631.
Lee, C., Kim, J., & Yoon, J. (2011). Inactivation of MS2 bacteriophage by streamer corona discharge in water. Chemosphere, 82, 1135–1140.
Lewis, D., & Metcalf, T. G. (1988). Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water, and sediment samples. Applied and Environmental Microbiology, 54(8), 1983–1988.
Lukasik, J., Scott, T. M., Andryshak, D., & Farrah, S. R. (2000). Influence of salts on virus adsorption to microporous filters. Applied and Environmental Microbiology, 66(7), 2914–2920.
Lute, S., Aranha, H., Tremblay, D., Liang, D., Ackermann, H.-W., Chu, B., Moineau, S., & Brorson, K. (2004). Characterization of coliphage PR772 and evaluation of its use for virus filter performance testing. Applied and Environmental Microbiology, 70(8), 4864–4871.
Mattison, K., Brassard, J., Gagné, M. J., Ward, P., Houde, A., Lessard, L., Simard, C., Shukla, A., Pagotto, F., Jones, T. H., & Trottier, Y. L. (2009). The feline calicivirus as a sample process control for the detection of food and waterborne RNA viruses. International Journal of Food Microbiology, 132, 73–77.
Méndez, J., Audicana, A., Isern, A., Llaneza, J., Moreno, B., Tarancón, M. L., Cofre, J., & Lucena, F. (2004). Standardised evaluation of the performance of a simple membrane filtration–elution method to concentrate bacteriophages from drinking water. Journal of Virology Methods, 117, 19–25.
Mesquita, M. M. F., Stimson, J., Chae, G.-T., Tufenkji, N., Ptacek, C. J., Blowes, D. W., & Emelko, M. B. (2010). Optimal preparation and purification of PRD1-like bacteriophages for use in environmental fate and transport studies. Water Research, 44, 1114–1125.
Morales-Morales, H. A., Vidal, G., Olszewski, J., Rock, C. M., Dasgupta, D., Oshima, K. H., & Smith, G. B. (2003). Optimization of a reusable hollow-fiber ultrafilter for simultaneous concentration of enteric bacteria, protozoa, and viruses from water. Applied and Environmental Microbiology, 69, 4098–4102.
Muller, J. E., Bessaud, M., Huang, Q. S., Martinez, L. C., Barril, P. A., Morel, V., Balanant, J., Bocacao, J., Hewitt, J., Gessner, B. D., Delpeyroux, F., & Nates, S. V. (2009). Environmental poliovirus surveillance during oral poliovirus vaccine and inactivated poliovirus vaccine use in Córdoba Province, Argentina. Applied and Environmental Microbiology, 75(5), 1395–1401.
Oshima, K. H. (2001). Efficient and predictable recovery of viruses and Cryptosporidium parvum oocysts from water by ultrafiltration systems. Technical Completion Report, New Mexico Water Research Resources Institute, New Mexico State University.
Park, G. W., Linden, K. G., & Sobsey, M. D. (2011). Inactivation of murine norovirus, feline calicivirus and echovirus 12 as surrogates for human norovirus (NoV) and coliphage (F+) MS2 by ultraviolet light (254 nm) and the effect of cell association on UV inactivation. Letters in Applied Microbiology, 52, 162–167.
Paul, J. H., Jiang, S. C., & Rose, J. B. (1991). Concentration of viruses and dissolved DNA from aquatic environments by vortex flow filtration. Applied and Environmental Microbiology, 57, 2197–2204.
Perry, R., La Torre, J., Kelley, D., & Greemberg, J. (1972). On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochimica et Biophysica Acta, 262, 220–226.
Petrinca, A. R., Donia, D., Pierangeli, A., Gabrieli, R., Degener, A. M., Bonanni, E., Diaco, L., Cecchini, G., Anastasi, P., & Divizia, M. (2009). Presence and environmental circulation of enteric viruses in three different wastewater treatment plants. Journal of Applied Microbiology, 106(5), 1608–1617.
Polson, A., Potgieter, G. M., Largier, J. F., Mears, G. E. F., & Joubert, F. J. (1964). The fractionation of protein mixtures by linear polymers of high molecular weight. Biochimca et Biophysica Acta, 82, 463–475.
Prüss-Üstün, A., & Corvalán, C. (2006). Preventing disease through healthy environments. Towards an estimate of the environmental burden of disease. France: World Health Organization.
Pusch, D., Oh, D. Y., Wolf, S., Dumke, R., Schröter-Bobsin, U., Höhne, M., Röske, I., & Schreier, E. (2005). Detection of enteric viruses and bacterial indicators in German environmental waters. Archives of Virology, 150(5), 929–947.
Rajal, V. B., McSwain, B. S., Thompson, D. E., Leutenegger, C. M., Kildare, B. J., & Wuertz, S. (2007). Validation of hollow fiber ultrafiltration and real-time PCR using bacteriophage PP7 as surrogate for the quantification of viruses from water samples. Water Research, 41, 1411–1422.
Rhodes, E. R., Hamilton, D. W., See, M. J., & Wymer, L. (2011). Evaluation of hollow-fiber ultrafiltration primary concentration of pathogens and secondary concentration of viruses from water. Journal of Virological Methods, 176, 38–45.
Schroeder, E. D., Stallard, W. M., Thompson, D. E., Loge, F. J., Deshusses, M. A., & Cox, H. H. (2002). Management of pathogens associated with storm drain discharge. Davis, Division of Environmental Analysis, California Department of Transportation. California, US.
Sheih, Y. S., Baric, R. S., & Sobsey, M. D. (1997). Detection of low levels of enteric viruses in metropolitan and airplane sewage. Applied and Environmental Microbiology, 63(11), 4401–4407.
Skraber, S., Gassilloud, B., & Gantzer, C. (2004). Comparison of coliforms and coliphages as tools for assessment of viral contamination in river water. Applied and Environmental Microbiology, 70(6), 3644–3649.
Sobsey, M. D., & Glass, J. S. (1984). Influence of water quality on enteric virus concentration by microporous filter methods. Applied and Environmental Microbiology, 47(5), 956–960.
Sobsey, M. D., & Hickey, A. R. (1985). Effects of humic and fulvic acids on poliovirus concentration from water by microporous filtration. Applied and Environmetal Microbiology, 49(2), 259–264.
Straub, T. M., & Chandler, D. P. (2003). Towards a unified system for detecting waterborne pathogens. Journal of Microbiological Methods, 53, 185–197.
Subramanian, S., Altaras, G. M., Chen, J., Hughes, B. S., Zhou, W., & Altaras, N. E. (2005). Pilot-scale adenovirus seed production through concurrent virus release and concentration by hollow fiber filtration. Biotechnology Progress, 21, 851–859.
Syngouna, V. I., & Chrysikopoulos, C. V. (2010). Interaction between viruses and clays in static and dynamic batch systems. Environmental Science and Technology, 44, 4539–4544.
Victoria, M., Guimarães, F., Fumian, T., Ferreira, F., Vieira, C., Leite, J. P., & Miagostovich, M. (2009). Evaluation of an adsorption–elution method for detection of astrovirus and norovirus in environmental waters. Journal of Virological Methods, 156, 73–76.
Weiss, S. A. (1980). Concentration of baboon endogenous virus in large-scale production by use of hollow-fiber ultrafiltration technology. Biotechnology and Bioengineering, 22, 19–31.
Winona, L. J., Ommani, A. W., Olszewski, J., Nuzzo, J. B., & Oshima, K. H. (2001). Efficient and predictable recovery of viruses from water by small scale ultrafiltration systems. Canadian Journal of Microbiolgy, 47, 1033–1041.
Acknowledgments
This research was part of the project PICT-Red 276/06 funded by the Agencia Nacional de Promoción de Ciencia y Técnica in Argentina (ANPCyT). This project was partially supported by NIH Grant D43 TW005718 funded by the Fogarty International Center and the National Institute of Environmental Health Sciences, USA. Hugo Ramiro Poma, Patricia Angélica Barril and Gisela Masachessi received fellowships from CONICET and María Dolores Blanco Fernández from ANPCyT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hugo Ramiro Poma and Verónica Beatriz Rajal shared first authorship.
Rights and permissions
About this article
Cite this article
Poma, H.R., Rajal, V.B., Blanco Fernández, M.D. et al. Evaluation of concentration efficiency of the Pseudomonas aeruginosa phage PP7 in various water matrixes by different methods. Environ Monit Assess 185, 2565–2576 (2013). https://doi.org/10.1007/s10661-012-2731-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2731-9