Abstract
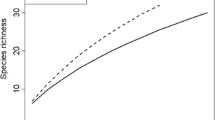
Mycorrhiza is the main spatial and temporal linkage between different constituents in a forest ecosystem. The functional compatibility and stress tolerance of ectomycorrhizal types is species specific, and therefore the information on the ectomycorrhizal community structure can add to the understanding of processes in forest ecosystems and can also be applied as tools for bioindication of pollution stress in forest soils. We have studied the effects of pollution (N and S) on trees and forest soils by: (1) quantification of ECM types diversity as in situ indicators in forest stands, (2) determination and quantification of pollution-sensitive or -insensitive ECM types as passive monitors, (3) root growth and development of ECM on nonmycorrhizal spruce seedlings, planted at the studied sites (active monitors), and (4) ECM infection (a bioassay based on mycorrhizal inoculum potential) of seedlings in an experimental set-up as ex situ testers. ECM species richness for Norway spruce trees (Picea abies) showed higher values in unpolluted sites than in polluted ones, while the differences were not significant for European beech trees (Fagus sylvatica). As pollution-sensitive or -insensitive ECM species in spruce forests, we suggest Hydnum rufescens (sensitive) and Paxillus involutus (unsensitive). Mycorrhizal potential in Norway spruce seedlings as a bioassay for soil N and S pollution was effective, and is suggested as an additional, standardized and widely comparable system in bioindication of soil pollution.
Similar content being viewed by others
References
Agerer, R. (1987–2002). Colour atlas of ectomycorrhizae. München: Einhorn-Verlag.
Agerer, R., & Rambold, G. (2004–2005). DEEMY-An information system for characterization and determination of ectomycorrhizae, http://www.deemy.de, Munich: Ludwig-Maximilians University.
Al Sayegh Petkovšek, S. (1997). Mycorrhizal potential of two differently polluted forest sites in the emission region of the Thermal Power Plant Šoštanj. Zbornik gozdarstva in lesarstva, 52, 323–350.
Al Sayegh Petkovšek, S. (2004). Biodiversity of types of ectomycorrhizae in fagus stands in differently polluted forest research plots. Zbornik gozdarstva in lesarstva, 75, 5–19.
Al Sayegh Petkovšek, S. (2005). Belowground ectomycorrhizal fungal communities at fagus stands in differently polluted forest research plots. Zbornik gozdarstva in lesarstva, 76, 5–38.
Al Sayegh Petkovšek, S., & Kraigher, H. (2003). Mycorrhizal potential of two forest research plots with respect to reduction of the emissions from the Thermal Power Plant Šoštanj. Acta Biologica Slovenica, 46, 9–16.
Arndt, U., Nobel, W., & Schweizer, B. (1987). Bioindikatoren-Möglichkeiten, Grenzen und neue Erkentnisse. Stuttgart: Ulmer.
Arnolds, E. (1988). The changing macromycete flora of the Netherlands. Transactions of the British Mycological Society, 90, 391–406.
Arnolds, E. (1991). Decline of ectomycorrhizal fungi in Europe. Agriculture, Ecosystems and Environment, 35, 209–244.
Atlas, R., & Bartha, R. (1981). Introduction to microbiology. Reading: Addison-Wesley Publishing Company.
Bakker, M. R. (1999). Fine root parameters as indicators of sustainability of forest ecosystems. Forest Ecology and Management, 122, 7–16.
Batič, F., & Kralj, T. (1995). Bioindikacija onesnaženosti ozračja v gozdovih z epifitskimi lišaji. Zbornik gozdarstva in lesarstva, 47, 5–56.
Dighton, J. (2003). Fungi in Ecosystem Processes (p. 432). New York: Marcel Dekker Inc.
Dighton, J., & Boddy, L. (1988). Role of fungi in nitrogen, phosphorus and sulphur cycling in temperate forest ecosystems. In L. Boddy, R. Marchant & D. J. Read (Eds.), Nitrogen, phosphorus and sulphur utilization by fungi. Symposium of the BMS (pp. 269–299). Cambridge: Cambridge Univ. Press.
Erland, S., & Taylor, A. F. S. (2002). Diversity of ectomycorrhizal fungal communities in relation to the abiotic environment. In M. van der Heijden & T. Sanders (Eds.), The ecology of ectomycorrhizas. Ecological studies Series, Volume 157, Chapter 7 (pp. 163–193). Springer Verlag.
Fellner, R. (1989). Mycorrhiza-forming fungi as bioindicators of air pollution. Agriculture, Ecosystems and Environment, 28, 115–120.
Fellner, R, & Peškova, V. (1995). Effects of industrial pollutants on ectomycorrhizal relationships in temperate forest. Canadian Journal of Botany, 73(Suppl. 1), 1310–1315.
Gadd, G. M. (2005). Microorganisms in toxic metal-polluted soils. In F. Buscot & Varma A. (Eds.), Microorganisms in soils: Roles in Genesis and functions, soil biology, volume 3 (pp. 325–356). Berlin Heidelberg: Springer-Verlag.
Gardes, M., & Bruns, T. D. (1993). ITS primers with enchanced specificity for basidiomycetes-application to the identification of ectomycorrhizae and rusts. Molecular Ecology, 2, 113–118.
Gianinazzi-Pearson, V. (1984). Host-fungus specifity, recognition and compatibility in Mycorrhizae. In D. P. S. Verma & T. Hohn (Eds.), Genes involved in Microbe-plant interactions (pp. 225–254). Wien: Springer-Verlag.
Grebenc, T. (2005). Tipi ektomikorize na bukvi (Fagus sylvatica L.) v naravnem in gospodarskem gozdu : doktorska disertacija = Types of ectomycorrhizae on beech (Fagus sylvatica L.) in natural and managed forest : doctoral dissertation. Ljubljana: 174 pp. http://www.digitalna-knjiznica.bf.uni-lj.si/dd_grebenc_tine.pdf.
Grebenc, T., & Kraigher, H. (2006). Types of ectomycorrhiza of mature beech and spruce at ozone-fumigated and control forest plots. EMAS (this volume).
Grebenc, T., Piltaver, A., & Kraigher, H. (2000). Establishment of a PCR-RFLP library for basidiomycetes, ascomycetes and their ectomycorrhizae on Picea abies (L.) Karst. Phyton (Horn, Austria), 40, 79–82.
Grimond, P. A. D. (1998). Taxotron user’s manual (p. 125). Paris: Institute Pasteur.
Hodge, A. (2004). The plastic plant: Root responses to heterogenous supplies of nutrients. New Phytologist, 162, 9–24.
Jaenike, J. (1991). Mass extinction of European fungi. Tree, 6, 174–175.
Kårén, O., Hogberg, N., Dahlberg, A., Jonsson, L., & Nylund, J. E. (1997). Inter- and intraspecific variation in the ITS region of rDNA of ectomycorrhizal fungi in Fennoscandia as detected by endonuclease analysis. New Phytologist, 136, 313–325.
Kovacs, G., Pausch, M., & Urban, A. (2000). Diversity of Ectomycorhizal Morphotypes and Oak Decline. Phyton (Horn, Austria), 40(4), 109–116.
Kraigher, H. (1999). Diversity of types of Ectomycorrhizae on Norway spruce in Slovenia. Phyton (Horn, Austria), 39, 199–202.
Kraigher, H., Agerer, R., & Javornik, B. (1995). Ectomycorrhiza of Lactarius lignyotus on Norway spruce, characterised by anatomical and molecular tools. Mycorrhiza, 5, 175–180.
Kraigher, H., Batič, F., & Agerer, R. (1996). Types of ectomycorrhizae and mycobioindication of forest site pollution. Phyton (Horn, Austria), 36, 115–120.
Kropaček, K., Kristinova M., Chemelikova, E., & Cudlin, P. (1989). The mycorrhizal inoculation potential of forest soils exposed to different pollutions stress. Agriculture, Ecosystems and Environment, 28, 217–277.
Simončič, P. (2000). Soil characteristics of the research plots in Zavodnje in the emmission area of the thermal power plant Šoštanj. In H. Kraigher & I. Smolej (Eds.), The Rhizosphere, Professional and Scientific Works 118 (pp. 77–88). Ljubljana: Slovenian Forestry Institute.
Simončič, P. (2001). Soil solution quality and soil characteristics with regard to clear cutting. Glasnik za šumske pokuse, 38, 159–166.
Simončič, P., Smolej, I., Kalan, P., Mavsar, R., & Levanič, T. (2004). Intensive monitoring in Slovenia (IMP-SI) : First annual report (2003) (p. 29). Ljubljana: Slovenian Forestry Institute; Wageningen: Alterra.
Smith, S. E., & Read, D. J. (1997). Mycorrhizal symbiosis (p. 605). Cambridge: Academic Press.
Smolej, I., Urbančič, M., Simončič, P., & Kutnar, L. (2000). Natural conditions and forest history of research plots. In H. Kraigher & I. Smolej (Eds.), The Rhizosphere, Professional and Scientific Works 118 (pp. 13–26). Slovenian Forestry Institute: Ljubljana.
Tausz, M., Batič, F., & Grill, D. (1996). Bioindication at forest sites-concepts, practice and outlook. Phyton (Horn,Austria), 36, 7–14.
Taylor, A. F. S., & Alexander, I. (2005). The ectomycorrhizal symbiosis: Life in the real world. Mycologist, 19, 102–112.
Taylor, A. F. S., Martin, F., & Read, D. J. (2000). Fungal diversity in ecto-mycorrhizal communities of Norway spruce (Picea abies (L.) Karst.) and Beech (Fagus sylvatica L.) along north-south transects in Europe. In E. D. Shulze (Ed.), Ecological studies, vol. 142 (pp. 343–365). Berlin Heilderberg New York: Springer.
Vidergar Gorjup, N., & Batič, F. (1999). Natural allotments, environmental pollution, and state of vegetation in Zasavje area. Gozdarski vestnik, 57, 80–91.
Vuković, N. (2003). Bioindication of stress in different forest sites with exposure of one year old spruce seedlings. Graduation thesis, Biotechnical Faculty, University of Ljubljana.
Waller, K., Agerer, R., Brand, F., Taylor, A. F. S., & Wanner, G. (1993). Piceirhiza oleiferans, eine neue Ektomykorrhizen-Art an Picea abies. Sendtnera, 1, 11–22.
White, T. J., Bruns, T., Lee, S., & Taylor J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In M. A. Innis, D. H. Gelfand, J. J. Sninsky & T. J. White (Eds.), PCR Protocols. A guide to methods and applications (pp. 315–322). San Diego: Academic Press.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kraigher, H., Al Sayegh Petkovšek, S., Grebenc, T. et al. Types of Ectomycorrhiza as Pollution Stress Indicators: Case Studies in Slovenia. Environ Monit Assess 128, 31–45 (2007). https://doi.org/10.1007/s10661-006-9413-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-006-9413-4