Abstract
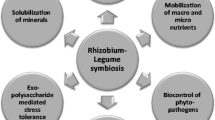
Common bean (Phaseolus vulgaris) plants are one of the most important legumes for human consumption due to its nutritional value and the fact it is rich in protein, iron, carbohydrates, and bioactive compounds. Diseases in common bean plants caused by soil-borne pathogens cause important economic losses in the world. Rhizobia is a well-known bacterial group that directly and indirectly promotes plant growth. The steps for biological nitrogen fixation performed in leguminous plants are well-known, as it is also known that the symbiosis between rhizobia and leguminous presents inhibitory effects against fungal diseases in plants. Thus, this work gathers information about the indirect effect in plant growth caused by rhizobia inoculation in common bean plants against the fungal pathogens Rhizoctonia solani, Fusarium oxysporum, Fusarium solani and Macrophomina phaseolina. Literature shows that the inoculation of common bean plants with different rhizobia isolates reduces or blocks the symptoms of disease caused by these phytopathogenic fungi. It is already known that rhizobia produce extracellular enzymes that hamper fungal development and induce physiological and molecular changes in plants. However, the specific mechanisms that govern these interactions and inhibitions still need to be clarified.
Similar content being viewed by others
References
Al-Askar, A. A., & Rashad, Y. M. (2010). Arbuscular mycorrhizal fungi: A biocontrol agent against Rashad common bean Fusarium root rot disease. Plant Pathology Journal, 9, 31–38.
Alves-Santos, F. M., Cordeiro-Rodrigues, L., Sayagués, J. M., Martín-Domínguez, R., García-Benavides, P., Crespo, M. C., Díaz-Mínguez, J. M., & Eslava, A. P. (2002). Pathogenicity and race characterization of Fusarium oxysporum f. Sp. phaseoli isolates from Spain and Greece. Plant Pathology, 51, 605–611.
Amira, M. B., Lopez, D., Mohamed, A. T., et al. (2017). Beneficial effect of Trichoderma harzianum strain Ths97 in biocontrolling Fusarium solani causal agent of root rot disease in olive trees. Biological Control, 110, 70–78.
Arora, N. K., Kang, S. C., & Maheshwari, K. D. (2001). Isolation of siderophore-producing strains of Rhizobium meliloti and their biocontrol potential against Macrophomina phaseolina that causes charcoal rot of groundnut. Current Science, 81, 673–677.
Barquero, M., Terrón, A., Velazquez, E., Gonzalez-Andres, F. (2016). Biocontrol of Fusarium oxysporum f. Sp. phaseoli and Phytophthora capsici with autochthonous endophytes in common bean and pepper in Castilla y León (Spain). Biological nitrogen fixation and beneficial plant-microbe interaction. Springer International Publishing, 221–235.
Basallote-Ureba, M. J., Vela-Delgado, M. D., Capote, N., Melero-Vara, J. M., López-Herrera, C. J., Prados-Ligero, A. M., & Talavera-Rubia, M. F. (2016). Control of Fusarium wilt of carnation using organic amendments combined with soil solarization, and report of associated Fusarium species in southern Spain. Crop Protection, 89, 184–192.
Bashan, Y., de-Bashan, L., Prabhu, S. R., & Hernandez, J. P. (2014). Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant and Soil, 378, 1–33.
Beatty, P. H., & Good, A. G. (2011). Future prospects for cereals that fix nitrogen. Science, 333, 416–417.
Beebe, S. E., Rao, I. M., Blair, M. W., & Acosta-Gallegos, J. A. (2013). Phenotyping common beans for adaptation to drought. Frontiers in Physiology, 4, 1–20.
Bhattacharjee, R. B., Jourand, P., Chaintreuil, C., Dreyfus, B., Singh, A., & Mukhopadhyay, S. N. (2012). Indole acetic acid and ACC deaminase-producing Rhizobium leguminosarum bv. Trifolii SN10 promote rice growth, and in the process undergo colonization and chemotaxis. Biology and Fertility of Soils, 48, 173–182.
Borras-Hidalgo, O., Caprari, C., Hernandez-Estevez, I., Lorenzo, G., & Cervone, F. (2012). A gene for plant protection: Expression of a bean polygalacturonase inhibitor in tobacco confers a strong resistance against Rhizoctonia solani and two oomycetes. Frontiers in Plant Science, 3, 1–6.
Broughton, W. J., Hernandez, G., Blair, M., Beebe, S., Gepts, P., & Vanderleyden, J. (2003). Beans (Phaseolus spp.) - model food legumes. Plant and Soil, 252, 55–128.
Buonassisi, A. J., Copeman, R. J., Pepin, H. S., & Eaton, G. W. (1986). Effect of Rhizobium spp. on Fusarium solani f. Sp. phaseoli. Canadian Journal of Plant Pathology, 8, 140–146.
Buttery, B. R., Tan, S. C., Drury, C. F., Park, S. J., Armstrong, R. J., & Park, K. Y. (1998). The effects of soil compaction, soil moisture and soil type on growth and nodulation of soybean and common bean. Canadian Journal of Plant Science, 78, 571–576.
Chakraboraty, U., & Purkayastha, R. P. (1984). Role of rhizobitoxine in protecting soybean roots from Macrophomina phaseolina infection. Canadian Journal of Microbiology, 30, 285–289.
Choudhary, S. R., & Sindhu, S. S. (2015). Suppression of Rhizoctonia solani root rot disease of clusterbean (Cyamopsis tetragonoloba) and plant growth promotion by rhizosphere bacteria. Plant Pathology Journal, 14, 48–57.
Coleman, J. J. (2016). The Fusarium solani species complex: Ubiquitous pathogens of agricultural importance. Molecular Plant Pathology, 17, 146–158.
CONAB. (2014) Acompanhamento da safra brasileira: grãos: safra /2015: décimo segundo levantamento, vol. 2. Available in: http://www.conab.gov.br.
Cross, H., Brick, M. A., Schwartz, H. F., Panella, L. W., & Byrne, P. F. (2014). Inheritance of resistance to fusarium wilt in two common bean races. Crop Science, 40, 954–958.
Dar, G. H., Zargar, M. Y., & Beigh, G. M. (1997). Biocontrol of Fusarium root rot in the common bean (Phaseolus vulgaris L.) by using symbiotic Glomus mosseae and Rhizobium leguminosarum. Microbial Ecology, 34, 74–80.
Das, K., Prasanna, R., & Saxena, A. K. (2017). Rhizobia: A potential biocontrol agent for soil-borne fungal pathogens. Folia Microbiologica, 62, 425–435.
de Borba, M. C., Garcés-Fiallos, F. R., & Stadnik, M. J. (2017). Reactions of black bean seedlings and adult plants to infection by Fusarium oxysporum f. Sp. phaseoli. Crop Protection, 96, 221–227.
de Jensen, C. E., Percich, J. A., & Graham, P. H. (2002). Integrated management strategies of bean root rot with Bacillus subtilis and Rhizobium in Minnesota. Field Crops Research, 74, 107–115.
Dean, R., Van Kan, J. A. L., Pretorius, Z. A., et al. (2012). The top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology, 13, 414–430.
Deshwal, V. K., Pandey, P., Kang, S. C., & Maheshwari, D. K. (2003). Rhizobia as a biological control agent against soil borne plant pathogenic fungi. Indian Journal of Experimental Biology, 41, 1160–1164.
Drapeau, R., Fortin, J. A., & Gagnon, C. (1973). Antifungal activity of Rhizobium. Canadian Journal of Botany, 51, 681–682.
Fageria, N. K., Melo, L. C., Ferreira, E. P. B., Oliveira, J. P., & Knupp, A. M. (2014). Dry matter, grain yield, and yield components of dry bean as influenced by nitrogen fertilization and rhizobia. Communication in Soil Sciences and Plant Analysis, 45, 111–125.
FAO, (2014). Food and agriculture Organization of the United Nations. Statistical databases. http://www.fao.org/faostat. Accessed 07 November 2017.
Filion, M., St-Arnaud, M., & Jabaji-Hare, S. H. (2003). Quantification of Fusarium solani f. Sp. phaseoli in mycorrhizal bean plants and surrounding mycorrhizosphere soil using real-time polymerase chain reaction and direct isolations on selective media. Phytopathology, 93, 229–235.
Gao, X., Jackson, T. A., Lambert, K. N., Li, S., Hartman, G. L., & Niblack, T. L. (2004). Detection and quantification of Fusarium solani f. Sp. glycines in soybean roots with real-time quantitative polymerase chain reaction. Plant Disease, 88, 2.
Gao, F. K., Dai, C. C., & Liu, X. Z. (2010). Mechanisms of fungal endophytes in plant protection against pathogens. African Journal of Microbiology Research, 4, 1346–1351.
Glick, B. R. (2012). Plant growth-promoting bacteria: Mechanisms and applications. Scientifica, 2012, 1–15.
Godoy-Lutz, G., Steadman, J. R., Higgins, B., & Powers, K. (2003). Genetic variation among isolates of the web blight pathogen of common bean based on PCR-RFLP of the ITS-rDNA region. Plant Disease, 87, 766–771.
Gopalakrishnan, S., Sathya, A., Vijayabharathi, R., Varshney, R. K., Gowda, C. L. L., & Krishnamurthy, L. (2015). Plant growth promoting rhizobia: Challenges and opportunities. 3 Biotech, 5, 355–377.
Granada, C., da Costa, P., Lisboa, B., Vargas, L. K., & Passaglia, L. M. P. (2013). Comparison among bacterial communities present in arenized and adjacent areas subjected to different soil management regimes. Plant and Soil, 373, 339–358.
Guerrero-González, M. L., Rodríguez-Kessler, M., Rodríguez-Guerra, R., González-Chavira, M., Simpson, J., Sanchez, F., & Jiménez-Bremont, J. F. (2011). Differential expression of Phaseolus vulgaris genes induced during the interaction with Rhizoctonia solani. Plant Cell Reports, 30, 1465–1473.
Hayashi, S., Gresshoff, P. M., & Ferguson, B. J. (2014). Mechanistic action of gibberellins in legume nodulation. Journal of Integrative Plant Biology, 56, 971–978.
Ilyas, M. B., & Sinclair, J. B. (1974). Effects of plant age upon development of necrosis and occurrence of intraxylern sclerotia in soybean infected with Macrophomina phaseolina. Phytopathology, 64, 156–157.
Kaur, S., Dhilllon, G. S., Brar, S. K., Vallad, G. E., Chand, R., & Chauhan, V. B. (2012). Emerging phytopathogen Macrophomina phaseolina: Biology, economic importance and current diagnostic trends. Critical Reviews in Microbiology, 38, 136–151.
Kelemu, S., Thomas, R. J., Moreno, C. X., & Ocampo, G. I. (1995). Strains of Bradyrhizobium from tropical forage legumes inhibit Rhizoctonia solani AG-1 in vitro. Australian Plant Patholology, 24, 168–172.
Kendig, S. R., Rupe, J. C., & Scott, H. D. (2000). Effect of irrigation and soil water stress on densities of Macrophomina phaseolina in soil and roots of two soybean cultivars. Plant Diseases, 84, 895–900.
Khan, S. N. (2007). Macrophomina phaseolina as causal agent for charcoal rot of sunflower. Mycopathology, 5, 111–118.
Kikot, G. E., Hours, R. A., & Alconada, T. M. (2009). Review contribution of cell wall degrading enzymes to pathogenesis of Fusarium graminearum: A review. Journal of Basic Microbiology, 49, 231–241.
Kucuk, C. (2013). In vitro antagonism of Rhizobium strains isolated from various legumes. Journal of Applied Biology Science, 7, 24–30.
Kumar, P., Pandey, P., Dubey, R. C., & Maheshwari, D. K. (2016). Bacteria consortium optimization improves nutrient uptake, nodulation, disease suppression and growth of the common bean (Phaseolus vulgaris) in both pot and field studies. Rhizosphere, 2, 13–23.
Lange, J., Xie, Z. P., Broughton, W. J., Vogeli-lange, R., & Boller, T. (1999). A gene encoding a receptor-like protein kinase in the roots of common bean is differentially regulated in response to pathogens, symbionts and nodulation factors. Plant Science, 142, 133–145.
Lehtonen, M. J., Somervuo, P., & Valkonen, J. P. T. (2008). Infection with Rhizoctonia solani induces defense genes and systemic resistance in potato sprouts grown without light. Phytopathology, 98, 1190–1198.
Margni, M., Rossier, D., Crettaz, P., & Jolliet, O. (2002). Life cycle impact assessment of pesticides on human health and ecosystems. Agriculture, Ecosystems & Environment, 93, 379–392.
Mayek-Pérez, N., Garcia-Espinosa, R., Lopez-Castaneda, C., Acosta-Callegos, J. A., & Simpson, J. (2002). Water relations, histopathology and growth of common bean (Phaseolus vulgaris L.) during pathogenesis of Macrophomina phaseolina under drought stress. Physiological and Molecular Plant Pathology, 60, 185–195.
Mazen, M. M., El-Batanony, N. H., Abd El-Monium, M. M., & Massoud, O. N. (2008). Cultural filtrate of Rhizobium spp. and arbuscular mycorrhiza are potential biological control agents against root rot fungal diseases of faba bean. Global Journal of Biotechnology & Biochemistry, 3, 32–41.
Meinhardt, L. W., Wulff, N. A., Bellato, C. M., & Tsai, S. M. (2002). Genetic analyses of Rhizoctonia solani isolates from Phaseolus vulgaris grown in the Atlantic rainforest region of São Paulo, Brazil. Fitopatologia Brasileira, 27, 259–267.
Mihail, J. D., & Taylor, S. J. (1995). Interpreting of variability among isolates of Macrophomina phaseolina in patogenicity, pycnidium production, and chlorate utilization. Canadian Journal of Botany, 73, 1596–1603.
Mora-Umaña, F., Cavallini, L.F.A. (1996). Combate biológico de Rhizoctonia solani mediante el empleo de Rhizobium leguminosarum biovar phaseoli en el campo. Reunión Anual del PCCMA 7, 23–30.
Moretti, A. (2009). Taxonomy of Fusarium genus: A continuous fight between lumpers and splitters. Matica Srpska Journal for Natural Sciences, 117, 7–13.
Mortier, V., Holsters, M., & Goormachtig, S. (2012). Never too many? How legumes control nodule numbers. Plant, Cell & Environment, 35, 245–258.
Naseri, B. (2013). Epidemics of Rhizoctonia root rot in association with biological and physicochemical properties of field soil in bean crops. Journal of Phytopathology, 161, 397–404.
Ndakidemi, P. A., Dakora, F. D., Nkonya, E. M., Ringo, D., & Mansoor, H. (2006). Yield and economic benefits of common bean (Phaseolus vulgaris) and soybean (Glycine max) inoculation in northern Tanzania. Australian Journal of Experimental Agriculture, 46, 571–577.
Nerey, Y., Pannecoucque, J., Hernandez, H. P., Diaz, M., Espinosa, R., de Vos, S., van Beneden, S., Herrera, L., & Höfte, M. (2010). Rhizoctonia spp. causing root and hypocotyl rot in Phaseolus vulgaris in Cuba. Journal of Phytopathology, 158, 236–243.
Olivares, J., Bedmar, E. J., & Sanjuán, J. (2013). Biological nitrogen fixation in the context of global change. Molecular Plant-Microbe Interactions, 26, 486–494.
Placinta, C. M., Mello, J. P. F. D., & Macdonald, A. M. C. (1999). A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Animal Feed Science and Technology, 78, 21–37.
Recchia, G. H., Caldas, D. G. G., Beraldo, A. L. A., da Silva, M. J., & Tsai, S. M. (2013). Transcriptional analysis of drought-induced genes in the roots of a tolerant genotype of the common bean (Phaseolus vulgaris L.). International Journal of Molecular Sciences, 14, 7155–7179.
Reitz, M., Rudolph, K., Schröder, I., Hallmann, J., & Sikora, R. A. (2000). Lipopolysaccharides of Rhizobium etli strain G12 act in potato roots as an inducing agent of systemic resistance to infection by the cyst nematode Globodera pallida. Applied and Environmental Microbiology, 66, 3515–3518.
Rusuku, G., Buruchara, R. A., Gatabazi, M., & Pastor-Corrales, M. A. (1997). Occurrence and distribution in Rwanda of Soilborne fungi pathogenic to the common bean. Plant Disease, 81, 445–449.
Sagolshemcha, R., Devi, I. N., & Singh, W. R. (2017). Plant growth promoting effect and biocontrol potential of Rhizobium spp. against Macrophomina phaseolina. International Journal of Current Microbiology and Applied Sciences, 6, 2695–2701.
Samavat, S., Samavat, S., Besharati, H., & Behboudi, K. (2011). Interactions of rhizobia cultural filtrates with Pseudomonas fluorescens on bean damping-off control. Journal of Agricultural Science and Technology, 13, 965–976.
Sasan, R. K., & Bidochka, M. J. (2013). Antagonism of the endophytic insect pathogenic fungus Metarhizium robertsii against the bean plant pathogen Fusarium solani f. Sp. phaseoli. Canadian Journal of Plant Pathology, 35, 288–293.
Seethapathy, P., Kurusamy, R., & Kuppusamy, P. (2017). Soil borne diseases of major pulses and their biological management. Innovative Farming, 2, 1–11.
Singh, S. P., & Schwartz, H. F. (2010). Breeding common bean for resistance to diseases: A review. Crop Science, 50, 2199–2223.
Srivastava, S., Kadooka, C., & Uchida, J. Y. (2018). Fusarium species as pathogen on orchids. Microbiological Research, 207, 188–195.
Tsror, L. (2010). Biology, epidemiology and management of Rhizoctonia solani on potato. Journal of Phytopathology, 158, 649–658.
Valentin Torres, S., Vargas, M. M., Godoy-Lutz, G., Porch, T. G., & Beaver, J. S. (2016). Isolates of Rhizoctonia solani can produce both web blight and root rot symptoms in common bean (Phaseolus vulgaris L.). Plant Disease, 2, 1351–1357.
Vital, D. A. L., Mejía, E. G., Dia, V. P., & Loarca-Piña, G. (2014). Peptides in common bean fractions inhibit human colorectal cancer cells. Food Chemistry, 157, 347–355.
Vollmann, J. (2016). Soybean versus other food grain legumes: A critical appraisal of the United Nations international year of pulses 2016. Journal of Land Management, Food Environment, 67, 17–24.
Wagner, S. C. (2012). Biological nitrogen fixation. Nature Education Know, 3, 15–23.
Xue, R., Wu, X., Wang, Y., Zhuang, Y., Chen, J., Wu, J., Ge, W., Wang, L., Wang, S., & Blair, M. W. (2017). Hairy root transgene expression analysis of a secretory peroxidase (PvPOX1) from common bean infected by Fusarium wilt. Plant Science, 260, 1–7.
Zhang, N., Donnell, K. O., Sutton, D. A., Nalim, F. A., Summerbell, R. C., Padhye, A. A., & Geiser, D. M. (2006). Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. Journal of Clinical Microbiology, 44, 2186–2190.
Acknowledgements
We thank Universidade do Vale do Taquari - Univates for financial support, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes/Brazil) for scolarships.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the present work was developed without any potential conflict of interest, with no human or animal participants. All authors read and approved the final version of this manuscript.
Rights and permissions
About this article
Cite this article
Schmidt, T.M., Thomé, A.H.E., Sperotto, R.A. et al. Effect of rhizobia inoculation on the development of soil-borne pathogens infecting common bean plants. Eur J Plant Pathol 153, 687–694 (2019). https://doi.org/10.1007/s10658-018-1600-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-018-1600-y