Abstract
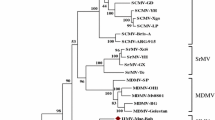
Cross reaction often occurs in serological detection for potyviruses that can lead to false positive results. Sequence alignment revealed that the N-terminal sequences of coat proteins (CP) are highly variable among potyviruses. Based on this finding, the preparation of a specific antibody against potyviruses is described here increasing the efficiency of detection and identification. A case study of Turnip mosaic virus (TuMV): a specific fragment “CP-50” (1–50 aa of CP) was obtained by prokaryotic expression and used for producing the polyclonal antibody. Test of specificity indicated that the prepared antibody “Anti-CP-50” strongly reacted with TuMV and is appropriate as a specific anti-TuMV antibody.




Similar content being viewed by others
Abbreviations
- TuMV:
-
Turnip mosaic virus
- CP:
-
Coat protein
- PAbs:
-
Polyclonal antibodies
- MAbs:
-
Monoclonal antibodies
- MW:
-
Molecular weight
- ELISA:
-
Enzyme linked immunosorbent assay
- GRAVY:
-
Grand average of hydropathicity.
References
Clark, M. F. (1981). Immunosorbent assays in plant pathology. Annual Review of Phytopathology, 19, 83–106.
Cooper, H. M., & Paterson, Y. (1995). Assays for antibody production. Current Protocols in Immunology, 2(4), 1–2.4.9.
Gasteiger, E., Hoogland, C., Gattiker, A., Duvaud, S., Wilkins, M. R., Appel, R. D., et al. (2005). Protein identification and analysis tools on the ExPASy server. In J. M. Walker (Ed.), The proteomics protocols handbook. Totowa: Humana Press.
Guruprasad, K., Reddy, B. V., & Pandit, M. W. (1990). Correlation between stability of a protein and its dipeptide composition: A novel approach for predicting in vivo stability of a protein from its primary sequence. Protein Engineering, 4, 155–161.
Hong, J., Li, D. B., & Zhou, X. P. (2001). Classification atlas of plant virus. Beijing: Science Press.
Hsu, H. (2009). Development of enzyme linked, tissue blot and dot blot immunoassays for plant virus detection. Plant Pathology, 508, 15–25.
ICTV. (2017). Virus Taxonomy: The Classification and Nomenclature of Viruses: The Online (10th) Report of the ICTV. http://talk.ictvonline.org/ictv-reports/ictv_online_report.
Kantrong, S., & Sako, N. (1993). Characterization of epitopes recognized by monoclonal antibodies to aphid transmissible and non-transmissible strains of Turnip mosaic virus. Archives of Virology, 133, 11–20.
Kantrong, S., Saunal, H., Briand, J. P., & Sako, N. (1995). A single amino acid substitution at N-terminal region of protein of Turnip mosaic virus alters antigenicity and aphid transmissibility. Virology, 140, 453–467.
Kyte, J., & Doolittle, R. F. (1982). A simple method for displaying the hydropathic character of a protein. Journal of Molecular Biology, 157, 105–132.
Naidu, R. A., & Hughes, J. d’A. (1997). Methods for the detection of plant virus diseases. International Institute of Tropical Agriculture, Ibadan, Nigeria, 45: 1–28.
Nguyen, H. D., Tomitaka, Y., Ho, S. Y., Duchêne, S., Vetten, H. J., Lesemann, D., et al. (2013). Turnip mosaic potyvirus probably first spread to eurasian brassica crops from wild orchids about 1000 years ago. PLoS One, 8(2), e55336.
Pearson, W. R., & Lipman, D. J. (1988). Improved tools for biological sequence comparison. Proceedings of the National Academy of Sciences, 85, 2444–2448.
Shukla, D. D., & Ward, C. W. (1989). Structure of potyvirus coat proteins and its application in the taxonomy of the potyvirus group. Advances in Virus Research, 36, 273–314.
Shukla, D. D., Strike, P. M., Tracy, S. L., Gough, K. H., & Ward, C. W. (1988). The N and C termini of the coat proteins of potyviruses are surface-located and the N terminus contains the major virus-specific epitopes. Journal of General Virology, 69, 1497–1508.
Shukla, D. D., Jilka, J., Tosic, M., & Ford, R. E. (1989a). A novel approach to the serology of potyviruses involving affinity purified polyclonal antibodies directed towards virus-specific N termini of coat proteins. Journal of General Virology, 70, 13–23.
Shukla, D. D., Tribbick, G., Mason, T. J., Hewish, D. R., Geysen, H. M., & Ward, C. W. (1989b). Localization of virus-specific and group-specific epitopes of plant potyviruses by systematic immunochemical analysis of overlapping peptide fragments. Proceedings of the National Academy of Sciences of the United States of America, 86, 8192–8196.
Thompson, S., Fraser, R. S., & Barnden, K. L. (1988). A beneficial effect of trypsin on the purification of Turnip mosaic virus (TuMV) and other potyviruses. Journal of Virological Methods, 20, 57–64.
Walsh, J. A., & Jenner, C. E. (2002). Turnip mosaic virus and the quest for durable resistance. Molecular Plant Pathology, 3, 289–300.
Zheng, G. H., Peng, D. W., Tong, Q. X., Zheng, Z. Z., & Ming, Y. L. (2017). Occurrence of Turnip mosaic virus in Phalaenopsis sp. in China. Journal of Plant Pathology, 99, 703–706.
Acknowledgements
The authors thank Prof. Chang Chin-an (Graduate Institute of Biochemical Sciences and Technology, Chaoyang University of Technology, Taichung, 41349, Taiwan) for providing experimental materials and technical assistance. We thank Peter Sol Reinach (Wenzhou Medical University) for providing language editing service of this manuscript. This work is supported by the grant from Fujian Provincial Natural Science Foundation of China (2011D002) and Xiamen Municipal Natural Science Foundation (3502Z20127015 & 3502Z20162014).
Funding
Fujian Provincial Natural Science Foundation of China (2011D002); Xiamen Municipal Natural Science Foundation (3502Z20127015 & 3502Z20162014).
Author information
Authors and Affiliations
Contributions
YLM and QXT conceived the experiments, and critically revised the manuscript. GHZ collected the samples. DWP and GHZ performed the experiments. ZZZ analyzed the data. DWP wrote the manuscript.
Corresponding author
Ethics declarations
This study was carried out following the guidelines and approval of the Laboratory Animal Ethics Committee of Wenzhou Medical University. The rabbits were anesthetized by 3% pentobarbital sodium (1 ml/kg) when collecting blood samples. After the experiments, rabbits were submitted to euthanasia using anesthetic overdose.
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Peng, D., Zheng, G., Zheng, Z. et al. High variability in the N terminus of coat protein among potyviruses and its advantage in producing a specific antibody. Eur J Plant Pathol 152, 385–393 (2018). https://doi.org/10.1007/s10658-018-1482-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-018-1482-z