Abstract
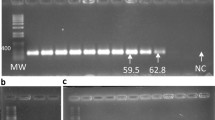
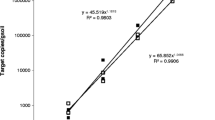
A qPCR approach was developed to specifically monitor in soils Fusarium graminearum, the main agent responsible for Fusarium Head Blight, and the biocontrol agent Gliocladium catenulatum J1446 (Prestop®). For both fungi, the amplification efficacy of standard curves obtained by mixing pure fungal DNA and soil background DNA was high (qPCR efficacy>96% with R2 > 0.97) with a linear range from 10−3 ng to 10 ng/μL. Our qPCR method allowed quantifying down to 1 μg of F. graminearum and G. catenulatum J1446 mycelium per g of soil. The strong correlation observed between fungal biomass and quantified DNA (R2 = 0.9927 and 0.9356 for F. graminearum and G. catenulatum J1446, respectively) supported the use of the primers to monitor both fungi in soils. Under our experimental conditions, the ability of Prestop® to reduce F. graminearum growth was significantly higher in autoclaved soil compared to living soils, suggesting that there is an antagonistic effect of the soil microbial communities. In contrast, G. catenulatum J1446 growth was mostly not affected by the presence of F. graminearum and was able to persist in both autoclaved and living soils after 15 days of incubation. These results indicate that our qPCR approach may be used to assess the success of soil colonization by a biocontrol agent and its control efficacy by monitoring the dynamics of the BCA and the targeted pathogen in soil.



Similar content being viewed by others
References
Alabouvette, C., Olivain, C., & Steinberg, C. (2006). Biological control of plant diseases: The European situation. European Journal of Plant Pathology, 114, 329–341.
Atoui, A., El Khoury, A., Kallassy, M., & Lebrihi, A. (2012). Quantification of Fusarium graminearum and Fusarium culmorum by real-time PCR system and zearalenone assessment in maize. International Journal of Food Microbiology, 154, 59–65.
Bateman, G. L., Gutteridge, R. J., Gherbawy, Y., Thomsett, M. A., & Nicholson, P. (2007). Infection of stem bases and grains of winter wheat by Fusarium culmorum and F. graminearum and effects of tillage method and maize-stalk residues. Plant Pathology, 56, 604–615.
Ben Amar, A., Oueslati, S., Ghorbel, A., & Mliki, A. (2012). Prediction and early detection of mycotoxigenic Fusarium culmorum in wheat by direct PCR-based procedure. Food Control, 23, 506–510.
Burgess, L. W., Backhouse, D., Summerell, B. A., & Swan, L. J. (2001). Crown rot of wheat. Pages 271-294 in. Fusarium: Paul E. Nelson. Memorial Symposium. American Phytopathology Society, St. Paul, MN.
Chatterton, S., & Punja, Z. K. (2009). Chitinase and β-1,3-glucanase enzyme production by the mycoparasite Clonostachys rosea f. catenulata against fungal plant pathogens. Canadian Journal of Microbiology, 55, 356–367.
Chatterton, S., & Punja, Z. K. (2011). Colonization of geranium foliage by Clonostachys rosea f. catenulata, a biological control agent of botrytis grey mould. Botany, 90, 1–10.
Chatterton, S., Jayaraman, J., & Punja, Z. K. (2008). Colonization of cucumber plants by the biocontrol fungus Clonostachys rosea f. catenulata. Biological Control, 46, 267–278.
Crane, J. M., Gibson, D. M., Vaughan, R. H., & Bergstrom, G. C. (2012). Iturin levels on wheat spikes linked to biological control of Fusarium head blight by Bacillus amyloliquefaciens. Phytopathology, 103, 146–155.
Davari, M., van Diepeningen, A. D., Babai-Ahari, A., Arzanlou, M., Najafzadeh, M. J., van der Lee, T. A. J., & de Hoog, G. S. (2012). Rapid identification of Fusarium graminearum species complex using rolling circle amplification (RCA). Journal of Microbiological Methods, 89, 63–70.
Demeke, T., Gräfenhan, T., Clear, R. M., Phan, A., Ratnayaka, I., Chapados, J., Patrick, S. K., Gaba, D., Lévesque, C. A., & Seifert, K. A. (2010). Development of a specific TaqMan® real-time PCR assay for quantification of Fusarium graminearum clade 7 and comparison of fungal biomass determined by PCR with deoxynivalenol content in wheat and barley. International Journal of Food Microbiology, 141, 45–50.
Dill-Macky, R., & Jones, R. K. (2000). The effect of previous crop residues and tillage on Fusarium head blight of wheat. Plant Disease, 84, 71–76.
Dyer, R. B., Kendra, D. F., & Brown, D. W. (2006). Real-time PCR assay to quantify Fusarium graminearum wild-type and recombinant mutant DNA in plant material. Journal of Microbiological Methods, 67, 534–542.
Fernandez, M. R., Huber, D., Basnyat, P., & Zentner, R. P. (2008). Impact of agronomic practices on populations of Fusarium and other fungi in cereal and noncereal crop residues on the Canadian prairies. Soil and Tillage Research, 100, 60–71.
Fredlund, E., Gidlund, A., Olsen, M., Börjesson, T., Spliid, N. H. H., & Simonsson, M. (2008). Method evaluation of Fusarium DNA extraction from mycelia and wheat for down-stream real-time PCR quantification and correlation to mycotoxin levels. Journal of Microbiological Methods, 73, 33–40.
Heise, J., Nega, M., Alawi, M., & Wagner, D. (2016). Propidium monoazide treatment to distinguish between live and dead methanogens in pure cultures and environmental samples. Journal of Microbiological Methods, 121, 11–23.
Horevaj, P., Milus, E. A., & Bluhm, B. h. (2011). A real-time qPCR assay to quantify Fusarium graminearum biomass in wheat kernels. Journal of Applied Microbiology, 111, 396–406.
Jiménez-Fernández, D., Montes-Borrego, M., Navas-Cortés, J. A., Jiménez-Díaz, R. M., & Landa, B. B. (2010). Identification and quantification of Fusarium oxysporum in planta and soil by means of an improved specific and quantitative PCR assay. Applied Soil Ecology, 46, 372–382.
Kernaghan, G., Reeleder, R. D., & Hoke, S. M. T. (2008). Quantification of Pythium populations in ginseng soils by culture dependent and real-time PCR methods. Applied Soil Ecology, 40, 447–455.
Khan, N. I., Schisler, D. A., Boehm, M. J., Slininger, P. J., & Bothast, R. J. (2001). Selection and evaluation of microorganisms for biocontrol of Fusarium head blight of wheat incited by Gibberella zeae. Plant Disease, 85, 1253–1258.
Kim, T. G., & Knudsen, G. R. (2016). Comparison of plate count, microscopy, and DNA quantification methods to quantify a biocontrol fungus. Applied Soil Ecology, 98, 285–288.
Kulik, T., Ostrowska, A., Buśko, M., Pasquali, M., Beyer, M., Stenglein, S., Załuski, D., Sawicki, J., Treder, K., & Perkowski, J. (2015). Development of an FgMito assay: A highly sensitive mitochondrial based qPCR assay for quantification of Fusarium graminearum sensu stricto. International Journal of Food Microbiology, 210, 16–23.
Kumar, A., Karre, S., Dhokane, D., Kage, U., Hukkeri, S., & Kushalappa, A. C. (2015). Real-time quantitative PCR based method for the quantification of fungal biomass to discriminate quantitative resistance in barley and wheat genotypes to fusarium head blight. Journal of Cereal Science, 64, 16–22.
Lee, H. J., & Ryu, D. (2017). Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: public health perspectives of their co-occurrence. Journal of Agricultural and Food Chemistry, 65, 7034–7051.
Le Floch, G., Tambong, J., Vallance, J., Tirilly, Y., Lévesque, A., & Rey, P. (2007). Rhizosphere persistence of three Pythium oligandrum strains in tomato soilless culture assessed by DNA macroarray and real-time PCR. FEMS Microbiology Ecology, 61, 317–326.
Li, M., Senda, M., Komatsu, T., Suga, H., & Kageyama, K. (2010). Development of real-time PCR technique for the estimation of population density of Pythium intermedium in forest soils. Microbiological Research, 165, 695–705.
Lievens, B., Brouwer, M., Vanachter, A. C. R. C., Cammue, B. P. A., & Thomma, B. P. H. J. (2006). Real-time PCR for detection and quantification of fungal and oomycete tomato pathogens in plant and soil samples. Plant Science, 171, 155–165.
Lv, X.-C., Li, Y., Qiu, W.-W., Wu, X.-Q., Xu, B.-X., Liang, Y.-T., Liu, B., Chen, S. J., Rao, P. F., & Ni, L. (2016). Development of propidium monoazide combined with real-time quantitative PCR (PMA-qPCR) assays to quantify viable dominant microorganisms responsible for the traditional brewing of Hong Qu glutinous rice wine. Food Control, 66, 69–78.
Mcquilken, Gemmell, & Lahdenperä. (2001). Gliocladium catenulatum as a potential biological control agent of damping-off in bedding plants. Journal of Phytopathology, 149, 171–178.
Moreno-Mesonero, L., Moreno, Y., Alonso, J. L., & Ferrús, M. A. (2016). DVC-FISH and PMA-qPCR techniques to assess the survival of Helicobacter pylori inside Acanthamoeba castellanii. Research in Microbiology, 167, 29–34.
Nannipieri, P., Ascher, J., Ceccherini, M. T., Landi, L., Pietramellara, G., & Renella, G. (2003). Microbial diversity and soil functions. European Journal of Soil Science, 54, 655–670.
Nicholson, P., Simpson, D. R., Weston, G., Rezanoor, H. N., Lees, A. K., Parry, D. W., & Joyce, D. (1998). Detection and quantification of Fusarium culmorum and Fusarium graminearum in cereals using PCR assays. Physiological and Molecular Plant Pathology, 53, 17–37.
Paavanen-Huhtala, S., Avikainen, H., & Yli-Mattila, T. (2000). Development of strain-specific primers for a strain of Gliocladium catenulatum used in biological control. European Journal of Plant Pathology, 106, 187–198.
Perez, C., Dill-Macky, R., & Kinkel, L. L. (2007). Management of soil microbial communities to enhance populations of Fusarium graminearum-antagonists in soil. Plant and Soil, 302, 53–69.
Punja, Z. K., & Yip, R. (2003). Biological control of damping-off and root rot caused by Pythium aphanidermatum on greenhouse cucumbers. Canadian Journal of Plant Pathology, 25, 411–417.
Ratti, C., Budge, G., Ward, L., Clover, G., Rubies-Autonell, C., & Henry, C. (2004). Detection and relative quantitation of soil-borne cereal mosaic virus (SBCMV) and Polymyxa graminis in winter wheat using real-time PCR (TaqMan®). Journal of Virological Methods, 122, 95–103.
Rose, S., Parker, M., & Punja, Z. K. (2003). Efficacy of biological and chemical treatments for control of Fusarium root and stem rot on greenhouse cucumber. Plant Disease, 87, 1462–1470.
Schisler, D. A., Khan, N. I., Boehm, M. J., & Slininger, P. J. (2002). Greenhouse and field evaluation of biological control of Fusarium head blight on durum wheat. Plant Disease, 86, 1350–1356.
Schroeder, K. L., Martin, F. N., de Cock, A. W. A. M., Lévesque, C. A., Spies, C. F. J., Okubara, P. A., et al. (2012). Molecular detection and quantification of Pythium species: Evolving taxonomy, new tools, and challenges. Plant Disease, 97, 4–20.
Verma, N., MacDonald, L., & Punja, Z. K. (2006). Inoculum prevalence, host infection and biological control of Colletotrichum acutatum: Causal agent of blueberry anthracnose in British Columbia. Plant Pathology, 55, 442–450.
Wagner, A. O., Praeg, N., Reitschuler, C., & Illmer, P. (2015). Effect of DNA extraction procedure, repeated extraction and ethidium monoazide (EMA)/propidium monoazide (PMA) treatment on overall DNA yield and impact on microbial fingerprints for bacteria, fungi and archaea in a reference soil. Applied Soil Ecology, 93, 56–64.
Wakelin, S. A., Warren, R. A., Kong, L., & Harvey, P. R. (2008). Management factors affecting size and structure of soil Fusarium communities under irrigated maize in Australia. Applied Soil Ecology, 39, 201–209.
Wegulo, S. N., Bockus, W. W., Nopsa, J. F. H., Peiris, K. H. S. & Dowell, F. E. (2013). Integration of fungicide application and cultivar resistance to manage Fusarium Head Blight in wheat. In M. Nita (Ed.), Fungicides-showcases of integrated plant disease management from around the world. InTech.
Woodhall, J. W., Adams, I. P., Peters, J. C., Harper, G., & Boonham, N. (2013). A new quantitative real-time PCR assay for Rhizoctonia solani AG3-PT and the detection of AGs of Rhizoctonia solani associated with potato in soil and tuber samples in great Britain. European Journal of Plant Pathology, 136, 273–280.
Zacharias, N., Kistemann, T., & Schreiber, C. (2015). Application of flow cytometry and PMA-qPCR to distinguish between membrane intact and membrane compromised bacteria cells in an aquatic milieu. International Journal of Hygiene and Environmental Health, 218, 714–722.
Acknowledgements
We gratefully thank Triskalia as project partners and the farmers for kindly giving us access to their field. We also thank the UBO Culture Collection for kindly providing us with fungal strains.
Funding
This work was supported by the Brittany Region [Grant #13008022 Rhisotox] and the French Association for Research and Technology ANRT [Grant #2014/108]. It was certified by the Foodstuff Cluster of the Future (Valorial).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authors confirm that this study is original and has not been published previously, and is not under consideration for publication elsewhere. All authors have approved the manuscript and agree with submission to this journal. This research does not contain any conflict of interest, nor research involving humans or animals.
Rights and permissions
About this article
Cite this article
Legrand, F., Picot, A., Cobo-Díaz, J.F. et al. Development of qPCR assays to monitor the ability of Gliocladium catenulatum J1446 to reduce the cereal pathogen Fusarium graminearum inoculum in soils. Eur J Plant Pathol 152, 285–295 (2018). https://doi.org/10.1007/s10658-018-1473-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-018-1473-0