Abstract
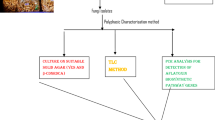
Aflatoxin contamination of tree nuts is a growing concern for pistachio producing countries. Development of competitive exclusion strategies through application of atoxigenic Aspergillus flavus isolates is a highly effective route of natural aflatoxin mitigation. Aflatoxin assays conducted on a high number of native A. flavus isolates are a first step to identify potential biological control isolates. Many cultural methods for the rapid and visual identification of atoxigenic A. flavus isolates have been described. The current study identified atoxigenic A. flavus isolates from Iranian pistachio orchards using and contrasting cultural, analytical and molecular methods. Ammonium vapour (AV) and fluorescence detection (FD), two rapid aflatoxin assays, were directly compared using various media preparations to screen 524 A. flavus isolates obtained from Iranian pistachio orchards. Percentages of false negatives were high using FD assays for all media preparations ranging from 13 to 15 %. This in contrast to AV assays. Here incidences of false negatives ranged from 0 % (using coconut agar medium) to 7.2 % (using potato dextrose agar). Aflatoxin-producing ability of all isolates was further confirmed using thin layer- and high-performance liquid chromatography. Sixty three atoxigenic A. flavus isolates were identified as atoxigenic in all assays. For these isolates, five loci across the aflatoxin biosynthesis cluster pathway were compared to identify genetic defects explaining atoxigenicity. Genetic deletions in at least one of five loci in the aflatoxin biosynthesis pathway were found for 97 % of isolates. Frequencies of atoxigenic strains ranged from 7.1 to 37.5 % with the lowest incidence detected in the Kerman province. Proper identification of atoxigenic isolates is considered a first step in the development of biological control strategies. Ability of identified isolates to competitively exclude aflatoxin-producing fungi has to be further investigated.


Similar content being viewed by others
References
Abbas, H. K., Shier, W. T., Horn, B. W., & Weaver, M. A. (2004a). Cultural methods for aflatoxin detection. Journal of Toxicology, 23, 295–319.
Abbas, H. K., Zablotowicz, R. M., Weaver, M. A., Horn, B. W., Xie, W., & Shier, W. T. (2004b). Comparison of cultural and analytical methods for determination of aflatoxin production by Mississippi Delta Aspergillus isolates. Canadian Journal of Microbiology, 50(3), 193–199.
Amaike, S., & Keller, N. P. (2011). Aspergillus flavus. Annual Review Phytopathology, 49, 107–133.
AOAC. (2000). Section 49.2.02 (AOAC Method 971.22) Preparation of standards. In Official methods of analysis, Seventeenth Edition. Gaithersburg, MD, USA, AOAC International.
Atehnkeng, J., Ojiambo, P. S., Donner, M., Ikotun, T., Sikora, R. A., Cotty, P. J., & Bandyopadhyay, R. (2008). Distribution and toxigenicity of Aspergillus species isolated from maize kernels from three agro-ecological zones in Nigeria. International Journal of Food Microbiology, 122(1), 74–84.
Bhatnagar, D., Ehrlich, K. C., & Cleveland, T. E. (2003). Molecular genetic analysis and regulation of aflatoxin biosynthesis. Applied Microbiology and Biotechnology, 61, 83–93.
Chang, P. K., Horn, B. W., & Dorner, J. W. (2005). Sequence breakpoints in the aflatoxin biosynthesis gene cluster and flanking regions in nonaflatoxigenic Aspergillus flavus isolates. Fungal Genetics and Biology, 42(11), 914–923.
Cheraghali, A. M., Yazdanpanah, H., Doraki, N., Abouhossain, G., Hassibi, M., Ali-Abadi, S., & Zamanian, F. (2007). Incidence of aflatoxins in Iran pistachio nuts. Food and Chemical Toxicology, 45(5), 812–816.
Cotty, P. J. (1990). Effect of atoxigenic strains of Aspergillus flavus on aflatoxin contamination of developing cotton seed. Plant Disease, 74, 233–235.
Cotty, P. J. (1997). Aflatoxin-producing potential of communities of Aspergillus section Flavi from cotton producing areas in the United States. Mycological Research, 101, 698–704.
Cotty, P. J., & Mellon, J. E. (2006). Ecology of aflatoxin producing fungi and biocontrol of aflatoxin contamination. Mycotoxin Research, 22, 110–117.
Cotty, P. J., Probst, C., & Jaime-Garcia, R. (2008). Etiology and management of aflatoxin contamination. In J. F. Leslie, R. Bandyopadhyay, & A. Visconti (Eds.), Mycotoxins: Detection methods, management, public health and agricultural trade (pp. 287–299). Oxfordshire: CAB International.
Davis, N. D., Diener, U. L., & Eldridgeand, D. W. (1966). Production of aflatoxins B1 and G1 by Aspergillus flavus in a semisynthetic medium. Applied Microbiology, 14, 378–380.
Davis, N. D., Iyer, S. K., & Diener, U. L. (1987). Improved method of screening for aflatoxin with a coconut agar medium. Applied and Environmental Microbiology, 53(7), 1593–1595.
Dini, A., Khazaeli, P., Roohbakhsh, A., Madadlou, A., Pourenamdari, M., Setoodeh, L., & Khodadadi, E. (2012). Aflatoxin contamination level in Iran’s pistachio nut during years 2009–2011. Food Control, 30(2), 540–542.
Donner, M., Atehnkeng, J., Sikora, R. A., Bandyopadhyay, R., & Cotty, P. J. (2009). Distribution of Aspergillus section Flavi in soils of maize fields in three agroecological zones of Nigeria. Soil Biology and Biochemistry, 41(1), 37–44.
Dorner, J. W. (2004). Biological control of aflatoxin contamination of crops. Journal of Toxicology, 23, 425–450.
Dorner, J. W. (2008). Management and prevention of mycotoxins in peanuts. Food Additives and Contaminants: Part A, Chemistry, Analysis, Control, Exposure and Risk Assessment, 25(2), 203–208.
Doster, M. A., & Michailides, T. J. (1994). The development of early split pistachio nuts and their contamination by molds, aflatoxins, and insects. Acta Horticulturae, 419, 359–364.
Fente, C. A., Ordaz, J. J., Vazquez, B. I., Franco, C. M., & Cepeda, A. (2001). New additive for culture media for rapid identification of aflatoxin-producing Aspergillus strains. Applied and Environmental Microbiology, 67(10), 4858–4862.
Gourama, H., & Bullerman, L. B. (1995). Aspergillus flavus and Aspergillus parasiticus: aflatoxigenic fungi of concern in foods and feeds: a review. Journal of Food Protection, 58(12), 1395–1404.
Hara, S., Fennel, D. I., & Hesseltine, C. W. (1974). Aflatoxin-producing strains of Aspergillus flavus detected by fluorescence of agar medium under ultraviolet light. Applied Environmental Microbiology, 27(6), 1118–1123.
Jaime-Garcia, R., & Cotty, P. J. (2006). Spatial relationships of soil texture and crop rotation to Aspergillus flavus community structure in south Texas. Phytopathology, 96, 599–607.
Klich, M. A. (2002). Identification of common Aspergillus species. Utrecht: Centraalbureau voor Schimmelcultures.
Mehl, H. L., Jaime, R., Callicott, K. A., Probst, C., Garber, N. P., Ortega-Beltran, A., Grubisha, L. C., & Cotty, P. J. (2012). Aspergillus flavus diversity on crops and in the environment can be exploited to reduce aflatoxin exposure and improve health. Annals of the New York Academy of Sciences, 1273, 7–17.
Michailides, T. J., Doster, M., Cotty, P. J., Morgan, D., Bockler, L. Felt, D. & Reyes, H. (2007). Aflatoxin control in pistachio: Biocontrol using the atoxigenic strain AF36, survival of AF36 and EUP status. Resource document. Proceeding of the 2007 annual multicrop aflatoxin/fumonisin elimination and fungal genomics workshop. http://www.ars.usda.gov/SP2UserFiles/Place/53254100/AEW/AEW2007.pdf. Accessed 3 Aug 2014.
Moradi, M., & Hokmabadi, H. (2011). Control of mycotoxin Bioactives in nuts: Farm to fork. In O. Tokusoglu & C. Hal III (Eds.), Fruit and cereal bioactives, sources, chemistry, and applications (pp. 291–315). Turkey: CRC Press.
Moradi, M., & Javanshah, A. (2006). Distribution of aflatoxin in processed pistachio nut terminals. Acta Horticulturae (ISHS), 726, 431–436.
Moradi, M., Oerke, E.-C., Steiner, U., Tesfaye, D., Schellander, K., & Dehne, H. W. (2010). Microbiological and Sybr®Green Real-Time PCR detection of major Fusarium Head Blight pathogens on wheat ears. Microbiology, 79(5), 646–654.
Nasir, M. S., & Jolley, M. E. (2002). Development of a fluorescence polarization assay for the determination of aflatoxins in grains. Journal of Agricultural and Food Chemistry, 50(11), 3116–3121.
O’Brian, G. R., Georgina, D. R., Wilkinson, J. R., Yu, J., Abbas, H. K., Bhatnagar, D., Cleveland, T. E., Nierman, W., & Payne, G. A. (2007). The effect of elevated temperature on gene transcription and aflatoxin biosynthesis. Mycologia, 99(2), 232–239.
Okoth, S., Nyongesa, B., Ayugi, V., Kang’ethe, E., Korhonen, H., & Joutsjoki, V. (2012). Toxigenic potential of Aspergillus species occurring on maize kernels from two agro-ecological zones in Kenya. Toxins, 4(11), 991–1007.
Park, D. L. (2002). Effect of processing on aflatoxin. Advances in Experimental Medicine and Biology, 504, 173–179.
Preacher, K. J. (2001). Calculation for the Chi-square test. An interactive calculation tool for chi-square tests of goodness of fit and independence. Ohio State University: http://www.quantpsy.org/-chisq/chisq.htm. Accessed 1 July 2014.
Probst, C., & Cotty, P. J. (2012). Relationship between in vitro and in vivo aflatoxin production. Reliable prediction of fungal ability to contaminate maize with aflatoxins. Fungal Biology, 116, 503–510.
Raper, K. B., & Fennell, D. I. (1965). The genus Aspergillus. New York: Springer.
Ritter, A. C., Hoeltz, M., & Noll, I. B. (2009). Toxigenic potential of Aspergillus flavus tested in different culture conditions. Ciência e Tecnologia de Alimentos, 31(3), 623–628.
Saito, M., & Machida, S. (1999). A rapid identification method for aflatoxin-producing strains of Aspergillus flavus and A. parasiticus by ammonia vapor. Mycoscience, 40, 205–208.
Sardiñas, N., Vázquez, C., Gil-Serna, J., González-Jaén, M. T., & Patiño, B. (2011). Specific detection and quantification of Aspergillus flavus and Aspergillus parasiticus in wheat flour by SYBR® Green quantitative PCR. International Journal of Food Microbiology, 145(1), 121–125.
Shier, W. T., Lao, Y., Steele, T. W., & Abbas, H. K. (2005). Yellow pigments used in rapid identification of aflatoxin-producing Aspergillus strain are anthraquinones associated with the aflatoxin biosynthetic pathway. Biorganic Chemistry, 33, 426–438.
Wei, D., & Jong, S. (1986). Production of aflatoxins by strains of the Aspergillus flavus group maintained in ATCC. Mycopathologia, 93, 19–24.
Williams, J. H., Phillips, T. D., Jolly, P. E., Stiles, J. K., Jolly, C. M., & Aggarwal, D. (2004). Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. American Journal of Clinical Nutrition, 80, 1106–1122.
Wiseman, H. G., Jacobson, W. C., & Harmeyer, W. C. (1967). Note on removal of pigments from chloroform extracts of aflatoxin cultures with copper carbonate. Journal of the Association of Official Analytical Chemists, 50, 982–983.
Yazdani, D., Zainal-Abidin, M. A., Tan, Y. H., & Kamaruzaman, S. (2010). Evaluation of the detection techniques of toxigenic Aspergillus isolates. African Journal of Biotechnology, 9(45), 7654–7659.
Zablotowicz, R. M., Abbas, H. K., & Locke, M. A. (2007). Population ecology of Aspergillus flavus associated with Mississippi Delta soils. Food Additives and Contaminants, 24(10), 1102–1108.
Zheng, Z., Saghaian, S., & Reed, M. (2012). Factors affecting the export demand for U.S. pistachios. Agribusiness Management Review, 15(3), 139–154.
Acknowledgments
We would like to express our special thanks to Dr. Peter J Cotty and Dr. Giuseppe Cozzi for their technical support and helpful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fani, S.R., Moradi, M., Probst, C. et al. A critical evaluation of cultural methods for the identification of atoxigenic Aspergillus flavus isolates for aflatoxin mitigation in pistachio orchards of Iran. Eur J Plant Pathol 140, 631–642 (2014). https://doi.org/10.1007/s10658-014-0499-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-014-0499-1