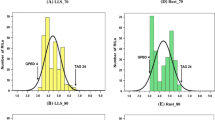
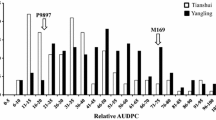
Abstract
The winter wheat lines Luke and AQ24788-83 are respectively susceptible and slow-rusting at tillering stage to yellow (stripe) rust, caused by Puccinia striiformis f. sp. tritici (Pst). A mapping population consisting of 206 recombinant inbred lines was developed from the cross Luke × AQ24788-83. These lines were evaluated at the tillering stage in the field trials for infection type (IT) and disease incidence (DI) and in greenhouse trials for IT and latent period (LP). A significant negative correlation was found between LP and DI. A genetic map with 473 marker loci was constructed and used for identifying QTL associated with LP and IT. Two QTL, QYr.cau-1BS and QYr.cau-5AS, were mapped on 1BS and 5AS respectively, explaining collectively up to 46.4 % of LP phenotypic variance. QYr.cau-5AS was clearly distinct, in terms of mapping position, from all six yellow rust resistance genes/QTL previously reported on 5A. QYr.cau-1BS could not be spatially differentiated from three (i.e. YrAlp, Yr15, and YrH52) of the six genes/QTL known on 1BS and centromere-vicinity regions, but was determined to be different from these three genes based on phenotype. The two QTL identified here, therefore, are likely to be novel to the currently known Pst resistance genes/QTL. A minor QTL on 3AL was detected to be associated with both IT and LP. Expression of quantitative resistance at early wheat growth stages and usefulness of the QTL are discussed for the wheat-Pst system.



Similar content being viewed by others
Abbreviations
- 1BS:
-
The short arm of chromosome 1B
- 5AS:
-
The short arm of chromosome 5A
- CIM:
-
Composite interval mapping
- DI:
-
Disease incidence
- cM:
-
centiMorgans
- IT:
-
Infection type
- LOD:
-
Logarithm of the odds
- LP:
-
Latent period
- MIM:
-
Multiple interval mapping
- Pst :
-
Puccinia striiformis Westend. f. sp. tritici Eriks.
- QTL:
-
Quantitative trait locus or quantitative loci, depending on context
- RIL:
-
Recombinant inbred line
- SSR:
-
Simple sequence repeat, microsatellite
References
Bariana, H. S., Parry, N., Barclay, I. R., Loughman, R., McLean, R. J., Shankar, M., et al. (2006). Identification and characterization of stripe rust resistance gene Yr34 in common wheat. Theoretical and Applied Genetics, 112, 1143–1148.
Bassam, B. J., Caetano-Anolles, G., & Gresshoff, P. M. (1991). Fast and sensitive silver staining of DNA in polyacrylamide gels. Analytical Biochemistry, 196, 80–83.
Basten, J. C., Wang, S., Gaffney, P., & Zeng, Z. B. (2003). Windows QTL cartographer, Version 2.0, statistical genetics. Raleigh: North Carolina State University.
Boukhatem, N., Baret, P. V., Mingeot, D., & Jacquemin, J. M. (2002). Quantitative trait loci for resistance against yellow rust in two wheat-derived recombinant inbred line populations. Theoretical and Applied Genetics, 104, 111–118.
Broers, L. H. M. (1997). Components of quantitative resistance to yellow rust in ten spring bread wheat cultivars and their relations with field assessments. Euphytica, 96, 215–223.
Broers, L. H. M., & Lopez-Atilano, R. M. (1994). A method of inoculating adult plants with urediniospores of Puccinia striiformis to measure components of resistance. Plant Disease, 78, 353–357.
Chen, W. Q., Wu, L. R., Liu, T. G., Xu, S. C., Jin, S. L., Peng, Y. L., et al. (2009). Race dynamics, diversity, and virulence evolution in Puccinia striiformis f. sp. tritici, the causal agent of wheat stripe rust in China from 2003 to 2007. Plant Disease, 93, 1093–1101.
Chen, X. M. (2005). Epidemiology and control of stripe rust (Puccinia striiformis f. sp. tritici) on wheat. Canadian Journal of Plant Pathology, 27, 314–337.
Chhuneja, P., Kaur, S., Garg, T., Ghai, M., Kaur, S., Prashar, M., et al. (2008). Mapping of adult plant stripe rust resistance genes in diploid A genome wheat species and their transfer to bread wheat. Theoretical and Applied Genetics, 116, 313–324.
de Vallavieille-Pope, C., Huber, L., Leconte, M., & Goyeau, H. (1995). Comparative effects of temperature and interrupted wet periods on germination, penetration, and infection of Puccinia recondita f. sp. tritici and P. striiformis on wheat seedlings. Phytopathology, 85, 409–415.
Fang, T., Campbell, K. G., Liu, Z. Y., Chen, X. M., Wan, A., Li, S., et al. (2011). Stripe rust resistance in the wheat cultivar Jagger is due to Yr17 and a novel resistance gene. Crop Science, 51, 2455–2465.
Fu, D., Uauy, C., Distelfeld, A., Blechl, A., Epstein, L., Chen, X. M., et al. (2009). A kinase-START gene confers temperature-dependent resistance to wheat stripe rust. Science, 323, 1357–1360.
Jagger, L. J., Newell, C., Berry, S. T., MacCormack, R., & Boyd, L. A. (2011). The genetic characterization of stripe rust resistance in the German wheat cultivar Alcedo. Theoretical and Applied Genetics, 122, 723–733.
Johnson, R. (1992). Reflections of a plant pathologist on breeding for disease, with emphasis on yellow rust and eyespot of wheat. Plant Pathology, 41, 239–254.
Krattinger, S. G., Lagudah, E. S., Spielmeyer, W., Singh, R. P., Huerta-Espino, J., McFadden, H., et al. (2009). A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science, 323, 1360–1363.
Lan, C. X., Liang, S. S., Zhou, X. C., Zhou, G., Lu, Q. L., Xia, X. C., et al. (2010). Identification of genomic regions controlling adult-plant stripe rust resistance in Chinese landrace Pingyuan 50 through bulked segregant analysis. Phytopathology, 100, 313–318.
Lander, E. S., Green, P., Abrahamson, J., Barlow, A., Daly, M. J., Lincoln, S. E., et al. (1987). MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics, 1, 174–181.
Lehman, J. S., & Shaner, G. (1997). Selection of populations of Puccinia recondita f. sp. tritici for shortened latent period on a partially resistant wheat cultivar. Phytopathology, 87, 170–176.
Lin, F., & Chen, X. M. (2007). Genetics and molecular mapping of genes for race-specific all-stage resistance and non-race-specific high-temperature adult-plant resistance to stripe rust in spring wheat cultivar Alpowa. Theoretical and Applied Genetics, 114, 1277–1287.
Line, R. F. (2002). Stripe rust of wheat and barley in North America: a retrospective historical review. Annual Review of Phytopathology, 40, 75–118.
Lowe, I., Jankuloski, L., Chao, S., Chen, X. M., See, D., & Dubcovsky, J. (2011). Mapping and validation of QTL which confer partial resistance to broadly virulent post-2000 North American races of stripe rust in hexaploid wheat. Theoretical and Applied Genetics, 123, 143–157.
Ma, H., & Singh, R. P. (1996). Expression of adult resistance to stripe rust at different growth stages of wheat. Plant Disease, 80, 375–379.
Mallard, S., Gaudet, D., Aldeia, A., Abelard, C., Besnard, A. L., Sourdille, P., et al. (2005). Genetic analysis of durable resistance to yellow rust in bread wheat. Theoretical and Applied Genetics, 110, 401–1409.
Milus, E. A., Seyran, E., & McNew, R. (2006). Aggressiveness of Puccinia striiformis f. sp. tritici isolates in the south-central United States. Plant Disease, 90, 847–852.
Murphy, L. R., Santra, D., Kidwell, K., Yan, G., Chen, X. M., & Campbell, K. G. (2009). Linkage maps of wheat stripe rust resistance genes Yr5 and Yr15 for use in marker-assisted selection. Crop Science, 49, 1786–1790.
Naz, A. A., Kunert, A., Flath, K., Pillen, K., & Léon, J. (2012). Advanced backcross quantitative trait locus analysis in winter wheat: dissection of stripe rust seedling resistance and identification of favorable exotic alleles originated from a primary hexaploid wheat (Triticum turgidum ssp. dicoccoides × Aegilops tauschii). Molecular Breeding. doi:10.1007/s11032-012-9710-2.
Park, R. F., & Rees, R. G. (1989). Expression of adult plant resistance and its effect on the development of Puccinia striiforrnis f. sp. tritici in some Australian wheat cultivars. Plant Pathology, 38, 200–208.
Parlevliet, J. E. (1975). Partial resistance of barley to leaf rust. Puccinia hordei. I. Effect of cultivar and development stage on latent period. Euphytica, 24, 21–27.
Peng, J. H., Fahima, T., Röder, M. S., Li, Y. C., Grama, A., & Nevo, E. (2000). Microsatellite high-density mapping of the stripe rust resistance gene YrH52 region on chromosome 1B and evaluation of its marker-assisted selection in the F2 generation in wild emmer wheat. New Phytologist, 146, 141–154.
Pestsova, E., Ganal, M. W., & Röder, M. S. (2000). Isolation and mapping of microsatellite markers specific for the D genome of bread wheat. Genome, 43, 689–697.
Qayoum, A., & Line, R. F. (1985). High-temperature, adult-plant resistance to stripe rust of wheat. Phytopathology, 75, 1121–1125.
Röder, M. S., Korzun, V., Wendehake, K., Plaschke, J., Tixier, M. H., Leroy, P., et al. (1998). A microsatellite map of wheat. Genetics, 149, 2007–2023.
Rogers, S. O., & Bendich, A. J. (1985). Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Molecular Biology, 5, 69–76.
Rosewarne, G. M., Singh, R. P., Huerta-Espino, J., Herrera-Foessel, S. A., Forrest, K. L., Hayden, M. J., et al. (2012). Analysis of leaf and stripe rust severities reveals pathotype changes and multiple minor QTLs associated with resistance in an Avocet × Pastor wheat population. Theoretical and Applied Genetics, 124, 1283–1294.
Singh, R. P. (1992). Genetic association of leaf rust resistance gene Lr34 with adult plant resistance to stripe rust in bread wheat. Phytopathology, 82, 835–838.
Somers, D. J., Isaac, P., & Edwards, K. (2004). A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theoretical and Applied Genetics, 109, 1105–1114.
Song, Q. J., Fickus, E. W., & Cregan, P. B. (2002). Characterization of trinucleotide SSR motifs in wheat. Theoretical and Applied Genetics, 104, 286–293.
Sourdille, P., Singh, S., Cadalen, T., Brown-Guedira, G. L., Gay, G., Qi, L., et al. (2004). Microsatellite-based deletion bin system for the establishment of genetic-physical map relationships in wheat (Triticum aestivum L.). Functional and Integrated Genomics, 4, 12–25.
Stubbs, R. W. (1988). Pathogenicity analysis of yellow (stripe) rust of wheat and its significance in a global context. In N. W. Simmonds & S. Rajaram (Eds.), Breeding strategy for resistance to the rusts of wheat (pp. 23–38). Mexico: CIMMYT.
Wan, A., Zhao, Z., Chen, X., He, Z., Jin, S., Jia, Q., et al. (2004). Wheat stripe rust epidemic and virulence of Puccinia striiformis f. sp. tritici in China in 2002. Plant Disease, 88, 896–904.
Wang, L., Ma, J., Zhou, R., Wang, X., & Jia, J. (2002). Molecular tagging of the yellow rust resistance gene Yr10 in common wheat, P.I.178383 (Triticum aestivum L.). Euphytica, 124, 71–73.
Wellings, C. R. (2011). Global status of stripe rust: a review of historical and current threats. Euphytica, 179, 129–141.
Xu, X.-Y., Bai, G.-H., Carver, B. F., Shaner, G. E., & Hunger, R. M. (2005). Mapping of QTLs prolonging the latent period of Puccinia triticina infection in wheat. Theoretical and Applied Genetics, 110, 244–251.
Zadoks, J. C., Chang, T. T., & Konzak, C. F. (1974). A decimal code for the growth stages of cereals. Weed Research, 14, 415–421.
Zhang, Z. J. (1995). Evidence of durable resistance in nine Chinese land races and one Italian cultivar of Triticum aestivum to Puccinia striiformis. European Journal of Plant Pathology, 101, 405–409.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (30871612), the Commonweal Specialized Research Fund of China Agriculture (200903035), and Program for Changjiang Scholars and Innovative Research Team (IRT1042).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quan, W., Hou, G., Chen, J. et al. Mapping of QTL lengthening the latent period of Puccinia striiformis in winter wheat at the tillering growth stage. Eur J Plant Pathol 136, 715–727 (2013). https://doi.org/10.1007/s10658-013-0201-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-013-0201-z