Abstract
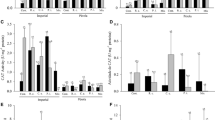
The ectomycorrhizal fungus Amanita vaginata can control damping off (Rhizoctonia solani) and promote growth of Pinus tabulaeformis seedlings. The aim of this study was to investigate whether reactive oxygen species and antioxidative enzymes play a role in preventing damping off in ectomycorrhizal roots. Two months after P. tabulaeformis roots were inoculated with A. vaginata, the roots were inoculated with R. solani. During the early stages (2–96 h) of R. solani infection, the quantity and localisation of hydrogen peroxide and the activities of superoxide dismutase and catalase were evaluated. A burst of hydrogen peroxide occurred in ectomycorrhizal roots and in non-ectomycorrhizal roots when attacked by R. solani. In ectomycorrhizal roots, hydrogen peroxide production peaked 12 h after R. solani inoculation, which coincided with an increase in the activity of superoxide dismutase and catalase, whereas in non-ectomycorrhizal roots, hydrogen peroxide production peaked 24 h after R. solani inoculation and did not coincide with changes in superoxide dismutase or catalase activity. The imbalanced activities of superoxide dismutase and catalase might cause excessive accumulation of hydrogen peroxide and consequent damage to cell walls. Electron microscopy revealed that there was a positive correlation between hydrogen peroxide levels and the number of amyloplasts, with seedlings inoculated with A. vaginata and/or R. solani showing higher levels. These results indicated that A. vaginata inoculation enhanced damping off resistance and stimulated seedling growth, which may be due to the activation of a burst of hydrogen peroxide and its scavenging enzymes and the production of biochemical substances such as amyloplasts.




Similar content being viewed by others
References
Abdel Latef, A. A. H. (2011). Influence of arbuscular mycorrhizal fungi and copper on growth, accumulation of osmolyte, mineral nutrition and antioxidant enzyme activity of pepper (Capsicum annuum L.). Mycorrhiza, 21, 495–503.
Bao, S. D. (2000). Soil and agricultural chemistry analysis (3rd ed.). Beijing, China: China Agriculture Press.
Baptista, P., Martins, A., Pais, M. S., Tavares, R. M., & Lino-Neto, T. (2007). Involvement of reactive oxygen species during early stages of ectomycorrhiza establishment between Castanea sativa and Pisolithus tinctorius. Mycorrhiza, 17, 185–193.
Bestwick, C. S., Brown, I. R., Bennett, M. H. R., & Mansfield, J. W. (1997). Localisation of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv. phaseolicola. The Plant Cell, 9, 209–221.
Branzanti, M. B., Rocca, E., & Pisi, A. (1999). Effect of ectomycorrhizal fungi on chestnut ink disease. Mycorrhiza, 9, 103–109.
De Deyn, G. B., Biere, A., van der Putten, W. H., Wagenaar, R., & Klironomos, J. N. (2009). Chemical defense, mycorrhizal colonization and growth responses in Plantago lanceolata L. Oecologia, 160, 433–442.
Dixon, R. A., & Lamb, C. J. (1990). Molecular communication in interactions between plants and microbial pathogens. Annual Review of Plant Physiology and Plant Molecular Biology, 41, 339–367.
Dumas-Gaudot, E., Gollotte, A., Cordier, C., Gianinazzi, S., & Gianinazzi-Pearson, V. (2000). Modulation of host defence systems. In Y. Kapulnik & D. D. J. Douds (Eds.), Arbuscular mycorrhizas: physiology and function (pp. 173–200). The Netherlands: Kluwer.
Fester, T., & Hause, G. (2005). Accumulation of reactive oxygen species in arbuscular mycorrhizal roots. Mycorrhiza, 15, 373–379.
Foreman, J., Demidchik, V., Bothwell, J. H. F., Mylona, P., Miedema, H., Torres, M. A., et al. (2003). Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature, 422, 442–446.
Gao, J. F. (2000). Experimental technology of phytophysiology. Xi’an China: World Book Publishing House.
García-Garrido, J. M., & Ocampo, J. A. (2002). Regulation of the plant defence response in arbuscular mycorrhizal symbiosis. Journal of Experimental Botany, 53, 1377–1386.
Garmendia, I., Aguirreolea, J., & Goicoechea, N. (2006). Defence-related enzymes in pepper roots during interactions with arbuscular mycorrhizal fungi and/or Verticillium dahliae. BioControl, 51, 293–310.
Iannone, M. F., Rosales, E. P., Groppa, M. D., & Benavides, M. P. (2010). Reactive oxygen species formation and cell death in catalase-deficient tobacco leaf disks exposed to cadmium. Protoplasma, 245, 15–27.
Joo, J. H., Bae, Y. S., & Lee, J. S. (2001). Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiology, 126, 1055–1060.
Lamb, C., & Dixon, R. A. (1997). The oxidative burst in plant disease resistance. Annual Review of Plant Physiology and Molecular Biology, 48, 251–275.
Lerat, S., Lapointe, L., Piché, Y., & Vierheilig, H. (2003). Variable carbon sink strength of different Glomus mosseae strains colonizing barley roots. Canadian Journal of Botany, 81, 886–889.
Liszkay, A., van der Zalm, E., & Schopfer, P. (2004). Production of reactive oxygen intermediates (O ·−2 , H2O2, and ·OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiology, 136, 3114–3123.
Martin, F., Duplessis, S., Ditengou, F., Lagrange, H., Voiblet, C., & Lapeyrie, F. (2001). Developmental cross talking in the ectomycorrhizal symbiosis: signals and communication genes. New Phytologist, 151, 145–154.
Martín-Pinto, P., Pajares, J., & Díez, J. (2006). In vitro effects of four ectomycorrhizal fungi, Boletus edulis, Rhizopogon roseolus, Laccaria laccata and Lactarius deliciosus on Fusarium damping off in Pinus nigra seedlings. New Forests, 32, 323–334.
Mysore, K. S., & Ryu, C.-M. (2004). Nonhost resistance: how much do we know? Trends in Plant Science, 9, 97–104.
Patterson, B. D., MacRae, E. A., & Ferguson, I. B. (1984). Estimation of hydrogen peroxide in plant extracts using titanium (IV). Analytical Biochemistry, 139, 487–492.
Patykowski, J. (2006). Role of hydrogen peroxide and apoplastic peroxidase in tomato-Botrytis cinerea interaction. Acta Physiologiae Plantarum, 28, 589–598.
Pellinen, R., Palva, T., & Kangasjärvi, J. (1999). Subcellular localization of ozone-induced hydrogen peroxide production in birch (Betula pendula) leaf cells. The Plant Journal, 20, 349–356.
Pozo, M. J., Cordier, C., Dumas-Gaudot, E., Gianinazzi, S., Barea, J. M., & Azcón-Aguilar, C. (2002). Localized versus systemic effect of arbuscular mycorrhizal fungi on defence responses to Phytophthora infection in tomato plants. Journal of Experimental Botany, 53, 525–534.
Pozo, M. J., Jung, S. C., López-Ráez, J. A., & Azcón-Aguilar, C. (2010). Impact of arbuscular mycorrhizal symbiosis on plant response to biotic stress: the role of plant defence mechanisms. In H. Koltai & Y. Kapulnik (Eds.), Arbuscular Mycorrhizas: Physiology and Function (pp. 193–207). Springer: Dordrecht, the Netherlands.
Sakai, A., Miyazawa, Y., Saito, C., Nagata, N., Takano, H., Hirano, H.-Y., et al. (1999). Amyloplast formation in cultured tobacco cells. III etermination of the timing of gene expression necessary for starch accumulation. Plant Cell Reports, 18, 589–594.
Salzer, P., Hebe, G., Reith, A., Zitterell-Haid, B., Stransky, H., Gaschler, K., et al. (1996). Rapid reactions of spruce cells to elicitors released from the ectomycorrhizal fungus Hebeloma crustuliniforme, and inactivation of these elicitors by extracellular spruce cell enzymes. Planta, 198, 118–126.
Sebastiana, M., Figueiredo, A., Acioli, B., Sousa, L., Pessoa, F., Baldé, A., et al. (2009). Identification of plant genes involved on the initial contact between Ectomycorrhizal symbionts (Castanea sativa—European chestnut and Pisolithus tinctorius). European Journal of Soil Biology, 45, 275–282.
Vierheilig, H., Steinkellner, S., Khaosaad, T., & Garcia-Garrido, J. M. (2008). mycorrhiza. In A. Varma (Ed.), The biocontrol ffect of mycorrhization on soilborne fungal pathogens and the autoregulation of the AM symbiosis: one mechanism, two effects? (pp. 307–320). Berlin Heidelberg: Springer–Verlag.
Wojtaszek, P. (1997). Oxidative burst: an early plant response to pathogen infection. Biochemical Journal, 322, 681–692.
Xavier, L. J. C., & Boyetchko, S. M. (2004). Arbuscular mycorrhizal fungi in plant disease control. In D. K. Arora (Ed.), Fungal biotechnology in agricultural, food, and environmental applications (pp. 183–194). New York: Dekke.
Zhang, R. Q., Tang, M., Chen, H., & Tian, Z. Q. (2011). Effects of ectomycorrhizal fungi on damping-off and induction of pathogenesis-related proteins in Pinus tabulaeformis seedlings inoculated with Amanita vaginata. Forest Pathology, 41, 262–269.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (30730073, 31170567), Program for Changjiang Scholars and Innovative Research Team in University of China (IRT1035) and the Ph. D. Programs Foundation of Education Ministry of China (20100204110033, 20110204130001). We also thank anonymous reviewers for their valuable suggestion to enhance the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, RQ., Tang, M. Role of hydrogen peroxide and antioxidative enzymes in Pinus tabulaeformis seedlings inoculated with Amanita vaginata and/or Rhizoctonia solani . Eur J Plant Pathol 134, 381–389 (2012). https://doi.org/10.1007/s10658-012-9996-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-012-9996-2