Abstract
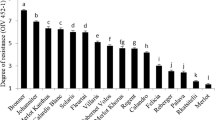
Cymoxanil has been used for over 30 years to control grape downy mildew (Plasmopara viticola) in European vineyards, prevalently in mixture with other fungicides active on this disease. In the 1990’s cases of P. viticola resistant to cymoxanil were detected using a leaf disc assay. In this study, we establish that the presence of only 1 % of resistant isolates in a P. viticola population will allow the detection of cymoxanil resistance in the leaf disc assay. A poor correlation (R = 0.194) was observed between the leaf disc assay and a whole- plant test for 38 P. viticola field populations collected in 2004. Over 60 % of these populations were characterized as fully sensitive in a whole-plant assay compared to 10 % in the leaf disc assay. Five P. viticola field isolates resistant to cymoxanil reverted to full sensitivity after six to nine transfers to untreated vines, indicating that cymoxanil resistance in P. viticola is unstable. Two European P. viticola populations sensitive to cymoxanil became resistant when transferred 12–14 times on vines treated with cymoxanil. In contrast, two populations originating from the USA and three monozoospore isolates from France retained full sensitivity to the fungicide after 13 cycles on cymoxanil-treated plants. Whole-plant experiments were conducted in the laboratory to compare the efficiency of spray programs to delay the development of cymoxanil resistance. Whereas the continuous use of cymoxanil alone quickly selected for resistance, the mixture of cymoxanil and folpet applied either continuously or in strict or block alternation effectively prevented the development of resistance over 10 generations of the fungus. These results demonstrate that resistance to cymoxanil in P. viticola can be managed with appropriate spray programs.



Similar content being viewed by others
References
Bleyer, G., Kassemeyer, H.-H., Krause, R., Viret, O., & Siegfried, W. (2008). “VitiMeteo Plasmopara” – Prognosemodell z ur Bekämpfung von Plasmopara viticola (Rebenperonospora) im Weinbau. Gesunde Pflanzen, 60, 91–100.
Brent, J.K. & Hollomon, D.W. (2007). Fungicide resistance in crop pathogens: How can it be managed? FRAC Monograph No. 1 (second, revised edition), from http://www.frac.info/frac/index.htm
Corio-Costet, M.-F., Dufour, M.-C., Cigna, J., Abadie, P., & Chen, W.-J. (2011). Diversity and fitness of Plasmopara viticola isolates resistant to QoI fungicides. European Journal of Plant Pathology, 129, 315–329.
Cowen, L. E., Kohn, L. M., & Anderson, J. B. (2001). Divergence in fitness and evolution of drug resistance in experimental populations of Candida albicans. Journal of Bacteriology, 10, 2971–2978.
Douchet, J.P., Absi, M., Hay, S.J.B., Mutan, L. & Villani, A. (1977). European results with DPX3217 a new fungicide for the control of grape downy mildew and potato late blight. Proc. Brit. Crop Prot. Conf. Pests and Diseases, 535–540
Genet, J.-L., & Vincent, O. (1999). Sensitivity of Eropean Plasmopara viticola populations to cymoxanil. Pesticide Science, 55, 129–136.
Genet, J.-L., Jaworska, G., & Deparis, F. (2006). Effect of dose rate and mixtures of fungicides on selection for QoI resistance in populations of Plasmopara viticola. Pest Management Science, 62, 188–194.
Genet, J.-L., Steva, H., Vincent, O., & Cazenave, C. (1997). A method for measuring the level of sensitivity of Plasmopara viticola populations to cymoxanil. Bulletin OEPP/EPPO, 27, 217–225.
Gisi, U., Waldner, M., Knaus, N., Dubuis, P. H., & Sierotzki, H. (2007). Inheritance of resistance to carboxylic acid amide (CAA) fungicides in Plasmopara viticola. Plant Pathology, 56, 199–208.
Gobbin, D., Rumbou, A., Linde, C. C., & Gessler, C. (2006). Population genetic structure of Plasmopara viticola after 125 years of colonization in European vineyards. Molecular Plant Pathology, 7(6), 519–531.
Gressel, J. (2011). Low pesticide rates may hasten the evolution of resistance by increasing mutation frequencies. Pest Management Science, 67, 253–257.
Grünwald, N. J., Sturbaum, A. K., Romero Montes, G., Serrano, E. G., Lozoya-Saldaña, H., & Fry, W. E. (2006). Selection for fungicide resistance within a growing season in field populations of Phytophthora infestans at the center of origin. Phytopathology, 96, 1397–1403.
Gullino, M. L., Mescalchin, E., & Mezzalama, M. (1997). Sensitivity to cymoxanil in populations of Plasmopara viticola in Northern Italy. Plant Pathology, 46, 729–736.
Harms, M., Führ, I., Lorenz, D., & Buchenauer, H. (2000). Investigations on the sensitivity of Plasmopara viticola to cymoxanil in the vine-growing area of Palatinate, Germany. Proceedings of the meeting on integrated control in viticulture, Florence, Italy, March 1–4, 1999. Bulletin OILB/SROP, 23, 41–44.
Herzog, J., & Schüepp, H. (1985). Three types of sensitivity of metalaxyl in Plasmopara viticola. Phytopath. Z, 114, 90–93.
Hobbelen, P. H. F., Paveley, N. D., & van den Bosch, F. (2011). Delaying selection for fungicide insensitivity by mixing fungicides at a low and high risk of resistance development: a modeling analysis. Phytopathology, 101, 1224–1233.
Howard, R. J., Ferrari, M. A., Genet, J-L. & Stidham, M. (2000). Biology of Curzate action against Plasmopara viticola infection of grape. Proc. AFPP, Sixieme Conf. Intn’l. sur les Maladies des Plantes, Tours, France
Li, H., Doazan, J. P., & Clerjeau, M. (1986). Etude de la variabilité et du pouvoir pathogène de Plasmopara viticola à l’égard de la vigne. I.- Comparaison de clones monosporocystes; rôle de l’hétérocaryose du parasite. Agronomie, 6(2), 187–194.
Langcake, P. (1981). Alternative chemical agents for controlling plant disease. Philosophical Transactions of the Royal Society of London - Series B: Biological Sciences, 295, 83–101.
Leroux, P., Fritz, R. & Despreaux, D. (1987). The mode of action of cymoxanil in Botrytis cinerea. In R. Greenhalgh & T. R. Roberts (eds.), (pp. 191–195). (Presented at Sixth IUPAC Congress of Pesticide Chemistry), Pesticide science and biotechnology. Blackwell Scientific Publications.
Matasci, C. L., Gobbin, D., Schärer, H.-J., Tamm, L., & Gessler, C. (2008). Selection for fungicide resistance throughout a growing season in populations of Plasmopara viticola. European Journal of Plant Pathology, 120, 79–83.
Pringle, A., & Taylor, J. W. (2002). The fitness of filamentous fungi. Trends in Microbiology, 10, 474–481.
Schüepp, H., & Herzog, J. (1986). Reliable method to produce single-zoospore cultures of Plasmopara viticola. Journal of Plant Diseases and Protection, 93, 30–32.
Shaner, G., & Finney, R. E. (1977). The effect of nitrogen fertilization on the expression of slow-mildewing resistance in knox wheat. The American Phytopathological Society, 67, 1051–1056.
Tellier, F., Fritz, R., Kerhoas, L., Ducrot, P.-H., Carlin-Sinclair, A., Einhorn, J., et al. (2009). Metabolism of fungicidal cyanooximes, cymoxanil and analogues in various strains of Botrytis cinerea. Pest Management, 65, 129–136.
Toffolatti, S. L., Prandato, M., Serrati, L., Sierotzki, H., Gisi, U., & Vercesi, A. (2011). Evolution of QoI resistance in Plasmopara viticola oospores. European Journal of Plant Pathology, 129, 331–338.
Van Den Bosch, F., Paveley, N., Shaw, M., Hobbelen, P., & Oliver, R. (2011). The dose rate debate: does the risk of fungicide resistance increase or decrease with dose? Plant Pathology, 60, 597–606.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Genet, JL., Jaworska, G. Characterization of European Plasmopara viticola isolates with reduced sensitivity to cymoxanil. Eur J Plant Pathol 135, 383–393 (2013). https://doi.org/10.1007/s10658-012-0094-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-012-0094-2