Abstract
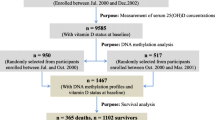
Oxidative stress (OS) has been found to be related to accelerated aging and many aging-related health outcomes. Recently, an epigenetic “mortality risk score” (MS) based on whole blood DNA methylation at 10 mortality-related CpG sites has been demonstrated to be associated with all-cause mortality. This study aimed to address the association between OS and MS, and to assess and compare their performance in the prediction of all-cause mortality. For 1448 participants aged 50–75 of the German ESTHER cohort study, the MS was derived from the DNA methylation profiles measured by Illumina HumanMethylation450K Beadchip and the levels of two urinary OS markers, 8-isoprostane (8-iso) and oxidized guanine/guanosine [including 8-hydroxy-2′-deoxyguanosine (8-oxo)], were measured by ELISA kits. Associations between OS markers and the MS were evaluated by linear and ordinal logistic regression models, and their associations with all-cause mortality were examined by Cox regression models. Both OS markers were associated with the MS at baseline. The 8-iso levels and MS, but not 8-oxo levels, were associated with all-cause mortality during a median follow-up of 15.1 years. Fully-adjusted hazard ratios (95% CI) were 1.56 (1.13–2.16) for the 4th quartile of 8-iso levels compared with the 1st, 1.71 (1.27–2.29) and 2.92 (2.03–4.18) for the moderate and high MS defined by 2–5 and > 5 CpG sites with aberrant methylation compared with a MS of 0–1, respectively. After controlling for 8-iso levels, the hazard ratios of MS remained essentially unchanged while the association of 8-iso levels with mortality was attenuated. This study demonstrates that OS is highly associated with the epigenetic MS, and the latter at the same time has a higher predictive value for all-cause mortality.




Similar content being viewed by others
References
Salmon AB, Richardson A, Perez VI. Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med. 2010;48(5):642–55. https://doi.org/10.1016/j.freeradbiomed.2009.12.015.
Salminen A, Ojala J, Kaarniranta K, Kauppinen A. Mitochondrial dysfunction and oxidative stress activate inflammasomes: impact on the aging process and age-related diseases. Cell Mol Life Sci. 2012;69(18):2999–3013. https://doi.org/10.1007/s00018-012-0962-0.
Schöttker B, Saum KU, Jansen EH, Boffetta P, Trichopoulou A, Holleczek B, et al. Oxidative stress markers and all-cause mortality at older age: a population-based cohort study. J Gerontol A Biol Sci Med Sci. 2015;70(4):518–24. https://doi.org/10.1093/gerona/glu111.
Schöttker B, Brenner H, Jansen EH, Gardiner J, Peasey A, Kubinova R, et al. Evidence for the free radical/oxidative stress theory of ageing from the CHANCES consortium: a meta-analysis of individual participant data. BMC Med. 2015;13(1):300. https://doi.org/10.1186/s12916-015-0537-7.
Stephens JW, Khanolkar MP, Bain SC. The biological relevance and measurement of plasma markers of oxidative stress in diabetes and cardiovascular disease. Atherosclerosis. 2009;202(2):321–9. https://doi.org/10.1016/j.atherosclerosis.2008.06.006.
Montuschi P, Collins JV, Ciabattoni G, Lazzeri N, Corradi M, Kharitonov SA, et al. Exhaled 8-isoprostane as an in vivo biomarker of lung oxidative stress in patients with COPD and healthy smokers. Am J Respir Crit Care Med. 2000;162(3 Pt 1):1175–7. https://doi.org/10.1164/ajrccm.162.3.2001063.
Kinnula VL, Ilumets H, Myllarniemi M, Sovijarvi A, Rytila P. 8-Isoprostane as a marker of oxidative stress in nonsymptomatic cigarette smokers and COPD. Eur Respir J. 2007;29(1):51–5. https://doi.org/10.1183/09031936.00023606.
Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′ -deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27(2):120–39. https://doi.org/10.1080/10590500902885684.
Gao X, Gào X, Zhang Y, Breitling LP, Schöttker B, Brenner H. Associations of self-reported smoking, cotinine levels and epigenetic smoking indicators with oxidative stress among older adults: a population-based study. Eur J Epidemiol. 2017;32(5):443–56. https://doi.org/10.1007/s10654-017-0248-9.
Mikeska T, Craig JM. DNA methylation biomarkers: cancer and beyond. Genes. 2014;5(3):821–64. https://doi.org/10.3390/genes5030821.
Hedman AK, Mendelson MM, Marioni RE, Gustafsson S, Joehanes R, Irvin MR et al. Epigenetic patterns in blood associated with lipid traits predict incident coronary heart disease events and are enriched for results from genome-wide association studies. Circ Cardiovasc Genet 2017;10(1). https://doi.org/10.1161/circgenetics.116.001487.
Zhang Y, Schöttker B, Florath I, Stock C, Butterbach K, Holleczek B, et al. Smoking-associated DNA methylation biomarkers and their predictive value for all-cause and cardiovascular mortality. Environ Health Perspect. 2016;124(1):67–74. https://doi.org/10.1289/ehp.1409020.
Zhang Y, Wilson R, Heiss J, Breitling LP, Saum KU, Schottker B, et al. DNA methylation signatures in peripheral blood strongly predict all-cause mortality. Nat Commun. 2017;8:14617. https://doi.org/10.1038/ncomms14617.
Gao X, Zhang Y, Schöttker B, Brenner H. Vitamin D status and epigenetic-based mortality risk score: strong independent and joint prediction of all-cause mortality in a population-based cohort study. Clin Epigenet. 2018;10(1):84. https://doi.org/10.1186/s13148-018-0515-y.
Zhang Y, Saum KU, Schöttker B, Holleczek B, Brenner H. Methylomic survival predictors, frailty, and mortality. Aging (Albany NY) 2018. https://doi.org/10.18632/aging.101392.
Gao X, Zhang Y, Mons U, Brenner H. Leukocyte telomere length and epigenetic-based mortality risk score: associations with all-cause mortality among older adults. Epigenetics. 2018;13(8):846–57. https://doi.org/10.1080/15592294.2018.1514853.
Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinform. 2012;13:86. https://doi.org/10.1186/1471-2105-13-86.
Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037–57. https://doi.org/10.1002/sim.3841.
Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30(10):1105–17. https://doi.org/10.1002/sim.4154.
Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–87. https://doi.org/10.1002/(SICI)1097-0258(19960229)15:4%3c361:AID-SIM168%3e3.0.CO;2-4.
Schöttker B, Brenner H, Jansen EH, Gardiner J, Peasey A, Kubinova R, et al. Evidence for the free radical/oxidative stress theory of ageing from the CHANCES consortium: a meta-analysis of individual participant data. BMC Med. 2015;13(1):300. https://doi.org/10.1186/s12916-015-0537-7.
Kjaer LK, Cejvanovic V, Henriksen T, Petersen KM, Hansen T, Pedersen O, et al. Cardiovascular and all-cause mortality risk associated with urinary excretion of 8-oxoguo, a biomarker for rna oxidation, in patients with type 2 diabetes: a prospective cohort study. Diabetes Care. 2017;40(12):1771–8. https://doi.org/10.2337/dc17-1150.
Masia M, Padilla S, Fernandez M, Rodriguez C, Moreno A, Oteo JA, et al. Oxidative stress predicts all-cause mortality in HIV-infected patients. PLoS ONE. 2016;11(4):e0153456. https://doi.org/10.1371/journal.pone.0153456.
Centers for Disease C, Prevention. Smoking-attributable mortality, years of potential life lost, and productivity losses–United States, 2000–2004. MMWR Morb Mortal Wkly Rep 2008;57(45):1226–8.
Risch A, Plass C. Lung cancer epigenetics and genetics. Int J Cancer. 2008;123(1):1–7. https://doi.org/10.1002/ijc.23605.
Dalaveris E, Kerenidi T, Katsabeki-Katsafli A, Kiropoulos T, Tanou K, Gourgoulianis KI, et al. VEGF, TNF-alpha and 8-isoprostane levels in exhaled breath condensate and serum of patients with lung cancer. Lung Cancer. 2009;64(2):219–25. https://doi.org/10.1016/j.lungcan.2008.08.015.
Shen J, Deininger P, Hunt JD, Zhao H. 8-Hydroxy-2′-deoxyguanosine (8-OH-dG) as a potential survival biomarker in patients with nonsmall-cell lung cancer. Cancer. 2007;109(3):574–80. https://doi.org/10.1002/cncr.22417.
Murtas D, Piras F, Minerba L, Ugalde J, Floris C, Maxia C, et al. Nuclear 8-hydroxy-2′-deoxyguanosine as survival biomarker in patients with cutaneous melanoma. Oncol Rep. 2010;23(2):329–35.
Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. https://doi.org/10.1186/gb-2013-14-10-r115.
Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49(2):359–67. https://doi.org/10.1016/j.molcel.2012.10.016.
Schwartz J, Weiss ST. Cigarette smoking and peripheral blood leukocyte differentials. Ann Epidemiol. 1994;4(3):236–42.
Gutierrez-Arcelus M, Lappalainen T, Montgomery SB, Buil A, Ongen H, Yurovsky A, et al. Passive and active DNA methylation and the interplay with genetic variation in gene regulation. Elife. 2013;2:e00523. https://doi.org/10.7554/eLife.00523.
Acknowledgements
The ESTHER study was supported by the Baden-Württemberg state Ministry of Science, Research and Arts (Stuttgart, Germany), the Federal Ministry of Education and Research (Berlin, Germany), and the Federal Ministry of Family Affairs, Senior Citizens, Women and Youth (Berlin, Germany). Measurements of the oxidative stress markers were partly funded by the German Research Foundation (DFG, Grant No.: SCHO 1545/3-1). Xu Gao and Xīn Gào are supported by the grant from the China Scholarship Council (CSC). The authors gratefully acknowledge contributions of DKFZ Genomics and Proteomics Core Facility, especially Melanie Bewerunge-Hudler and Matthias Schick, in the processing of DNA samples and performing the laboratory work, Dr. Jonathan Heiss for providing the estimation of leukocyte distribution and Ms. Chen Chen for the language assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gao, X., Gào, X., Zhang, Y. et al. Oxidative stress and epigenetic mortality risk score: associations with all-cause mortality among elderly people. Eur J Epidemiol 34, 451–462 (2019). https://doi.org/10.1007/s10654-019-00493-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-019-00493-7