Abstract
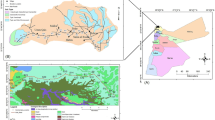
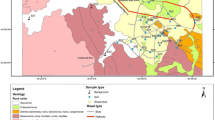
Heavy metal pollution caused by traffic activities is increasingly becoming a great threat to urban environmental quality and human health. In this paper, soils of Kerman urban and suburban areas were collected to assess the potential effects of traffic and other vehicle-related pollution by heavy metal accumulation in soils. Eighty-six samples were collected along streets and from residential and rural sectors, as well as vehicle-related workshops from depth of 0–5 and 15–20 cm and analyzed by flame atomic absorption spectrometry (FAAS) for heavy metals (Cd, Cr, Cu, Pb, Sn and Zn), as well as major elements (Al, Ca, Fe and Mn). Several hot-spot areas were identified in the composite geochemical maps produced based on Geographical Information System (GIS) technology. The majority of the hot-spot areas were identified to be vehicle-related workshops, fuel stations and road junctions. The most polluted hot-spot in the study area was located in soils close to a car battery processing workshop in the southwestern part of Kerman city, with concentrations of Cd (0.32 mg/kg), Cr (169 mg/kg), Cu (250 mg/kg), Pb (5,780 mg/kg), Sn (27.2 mg/kg) and Zn (178 mg/kg) of 1, 8.5, 8.3, 230, 13.5 and 3 times more than the relevant mean concentrations in natural soils, respectively. Traffic pollution has resulted in significant accumulation of heavy metals in soils and sediments, and that level of accumulation varied remarkably among elements. Based on X-ray diffraction analysis, most parts of soils and sediments of the Kerman basement consist of calcite and clay minerals. Abundance of clay minerals and medium to alkaline pH causes low mobility of heavy metals in soils of Kerman.






Similar content being viewed by others
References
Aghanabati, A. (2004). Geology of Iran. Tehran: Ministry of mines and Industries press.
Al-Khashman, O. A., & Shawabkeh, R. A. (2009). Metal distribution in urban soil around steel industry beside Queen Alia Airport, Jordan. Environmental Geochemistry and Health, 31(6), 717–726.
Alloway, B. J. (1995). Heavy metals in soils. London: Blackie Academic press.
Atapour, H., & Aftabi, A. (2002). Geomorphological, geochemical and geo-environmental aspects of karstification in the urban areas of Kerman city, southeastern Iran. Environmental Geology, 42(7), 783–792.
Balcerzak, M. (2002). Sample digestion methods for the determination of traces of precious metals by spectrometric techniques. Analytical Sciences, 18, 737–750.
Beckett, P. H. T. (1958). The soils of Kerman, South Persia. European Journal of Soil Science, 9(1), 20–31.
Berkowitz, B., Dror, I., & Yaron, B. (2008). Contaminant geochemistry: Interactions and transport in the subsurface environment. Springer, Berlin
Birke, M., & Rauch, U. (2000). Urban geochemistry: Investigations in the Berlin Metropolitan Area. Environmental Geochemistry and Health, 22(3), 233–248.
Botkin, D., & Keller, E. A. (2003). Environmental science, earth as a living planet. New York: Wiley.
Bottoms, S. (2000). Cu probraze process is proving a hot technology. Materials World, 8(2), 8–18.
Brümmer, G. W. (1986). Heavy metal species, mobility and availability in soils. The importance of chemical “speciation” in environmental processes. Berlin: Springer.
Cameron, K. C., Di, H. J., & McLaren, R. G. (1997). Is soil an appropriate dumping ground for our wastes? Australian Journal of Soil Research, 35(5), 995–1035.
Christoforidis, A., & Stamatis, N. (2009). Heavy metal contamination in street dust and roadside soil along the major national road in Kavala’s region, Greece. Geoderma, 151(3–4), 257–263.
Collins, S., Smalbone, K., & Briggs, D. (1995). A GIS approach to modeling small area variations in air pollution within a complex urban environment. London: Taylor and Francis.
Ebtekar, T. (2001). Collection of Mohammad Taqi Ebtekar's articles. Tehran: Environmental protection organization of Iran press.
Eby, G. N. (2004). Principles of environmental geochemistry. New York: Thomson, Ellis, S. & Mellor, A.
Evangelou, V. P. (1998). Environmental soil and water chemistry. New York: Wiley.
Facchinelli, A., Sacchi, E., Mallen, L., et al. (2000). Multivariate statistical and GIS-based approach to identify heavy metal sources in soil. Environmental Pollution, 114(3), 313–324.
Faure, G. (1992). Principles and application of inorganic geochemistry. New York: Wiley.
Gagnon, C., Turcotte, P., & Vigneault, B. (2009). Comparative study of the fate and mobility of metals discharged in mining and urban effluents using sequential extractions on suspended solids. Environmental Geochemistry and Health, 31(6), 657–671.
Graef, J. W. (1997). Getting the lead out. New York: Plenum Press.
Haal, M. L., Surje, P., Rouk, H., et al. (2008). Traffic as a source of pollution. Estonian Journal of Engineering, 14(1), 65–82.
Hamzeh, M. A. & Aftabi, A. (2006). Geochemical and environmental indicators in the urban areas of Kerman city, Kerman, Iran. Dissertation, Shahid Bahonar Univ. of Kerman & Inter. Center for Science and High Tech. and Environ. Sciences.
Harrison, R. M., Laxen, D. P. H., Wilson, S. J., et al. (1981). Chemical associations of lead, cadmium, copper and zinc in street dust and roadside soil. Environmental Science and Technology, 15(2), 1378–1383.
Kabata-Pendias, A. (2000). Trace elements in soils and plants (3rd ed.). Boca Raton, FL: CRC Press.
Langmuir, D. (1997). Aqueous environmental geochemistry. New Jersey: Prentice-Hall.
Merian, E., Anke, M., Ihnat, M., & Stoeppler, M. (2004). Elements and their compounds in the environment, occurrence, analysis and biological relevance. New York: VCH Verlag, Wiley
Mielke, H. W., Gonzales, C. R., Smith, M. K., Mielke, P. W., et al. (2000). Quantities and associations of lead, zinc, cadmium, manganese, chromium, nickel, vanadium and copper in fresh Mississippi delta alluvium and New Orleans alluvium soils. The Science of Total Environment, 246(2), 249–259.
Müller, G. (1979). Schwermetalle in den sedimenten des Rheins—Veränderungen seit 1971. Umschau, 79, 778–783.
NIOPDC (2010). http://www.niopdc.ir/HomePage.aspx.
O’Connor, B. H., & Jaklevic, J. M. (1981). Characterization of ambient aerosol particulate samples from the St. Louis Area by X-ray powder diffractometry. Atmospheric Environment, 15(4), 1681–1690.
Pilgrim, W., & Hughes, R. N. (1994). Lead, cadmium, arsenic and zinc in the ecosystem surrounding a lead smelter. Environmental Monitoring and Assessment, 32(1), 1–20.
Pilinis, C., & Seinfeld, J. H. (1987). Continued development of a general equilibrium model for inorganic multicomponent aerosol. Atmospheric Environment, 21(2), 2453–2466.
Pirrone, N., Costa, P., & Pacyna, J. M. (1999). Past, current and projected atmospheric emissions of trace elements in the Mediterranean region. Water, Science and Technology, 39(2), 1–7.
Radojevic, M. V., & Bashkin, N. (1999). Practical environmental analysis. New York: Royal Society of Chemistry Press.
Rahimi, H., Kalbasi, M., Hajrasouliha, S. H., et al. (2000). Pb soil pollution by vehicles around some of Iran highways. Agriculture and Nature Research Science, 14(3), 31–41.
Rea, A., Lindberg, S., Keeler, G., et al. (2001). Dry deposition and foliar leaching of mercury and selected trace elements in deciduous forest throughfall. Atmospheric Environment, 35(2), 3453–3462.
Reimann, C., & Caritat, P. (1998). Chemical elements in the environment: Fact sheets for the geochemist and environmental scientist (p. 398). Berlin: Springer.
Rule, J. H. (1999). Trace metal cation adsorption in soils: selective chemical extractions and biological availability. Studies in Surface Science and Catalysis, 120(2), 319–349.
Sayed, S. M., Ashour, E. A., Youssef, G. I., et al. (2003). Effect of sulfide ions on the corrosion behavior Al-brass and Cu10Ni alloys in salt water. Mathematics Chemistry and Physics, 78(3), 825–834.
Schumacher, M., Meneses, M., Granero, S., Llobet, J. M., Domingo, J. L., et al. (1997). Trace element pollution of soil collected near a municipal solid waste incinerator. Environmental Contamination and Toxicology, 59(4), 861–867.
Selinus, O., Alloway, B. J., Centeno, J. A., Finkelman, R. B. et al. (2005). Essentials of medical geology: impacts of natural environment on public health. Elsevier Academic Press, Burlington, USA, p 812.
Seyferth, D. (2003). The rise and fall of tetraethyl lead. Organometallics, 22(3), 5154–5178.
Simonson, R. W. (1995). Airborne dust and its significance to soil. Geoderma, 65(1), 1–43.
Surthland, R. A., Tolosa, C. A., Tack, F. M. G., Verloo, M. G., et al. (2000). Characterization of selected element concentrations and enrichment ratios in background and anthropogenically impacted roadside areas. Archive Environmental Contamination and Toxicology, 38(3), 428–438.
Thomas, V. (1995). The elimination of lead in gasoline. Annual Review Energy Environment, 20(2), 301–324.
Thomas, V., Socolow, R., Fanelli, J., Spiro, T., et al. (1999). Effects of reducing lead in gasoline, an analysis of the international experience. Environmental Science and Technology, 33(2), 3942–3947.
Thornton, I. (1991). Metal contamination of soils in urban areas, soil in urban environment. Oxford: Blackwell.
Tiller, K. G. (1992). Urban soils contamination in Australia. Australian Journal Soil Research, 30(6), 937–957.
Wedepohl, K. H. (1995). The composition of the continental crust. Geochimica et Cosmochimica Acta, 59, 1217–1232.
Westerlund, K. (2001). Metal emissions from Stockholm traffic—wear of brake linings. Reports from SLB-analysis no. 3: 2001, The Stockholm Environment and Health Protection Administration.
Wilcke, W., Muller, S., Kanchanakool, N., Zeeh, W., et al. (1998). Urban soil contamination in Bangkok: heavy metal and aluminum partitioning in topsoils. Geoderma, 86(3–4), 211–228.
Xiangdong, L., Siu-lan, L., Sze-chung, W., Wenzhong, S., Thornton, I., et al. (2004). The study of metal contamination in urban soils of Hong Kong using a GIS-based approach. Environmental Pollution, 129(1), 113–124.
Xiangdong, L., Wenzhong, S., Thornton, I., et al. (2006). Metal contamination in urban, suburban and country park soil of Hong Kong: A study based on GIS and multivariate statistic. Science of the Total Environment, 356(1–3), 45–61.
Zeng, Y. H., Zueng-Sang, C., Chen-Chi, T., Chun-Chi, T., et al. (2002). Digestion methods for total heavy metals in sediments and soils. Water, Air, and Soil Pollution, 141(1–4), 189–205.
Acknowledgments
This investigation was supported by Department of Geology, Faculty of Sciences, Shahid Bahonar University of Kerman. Partial support for this project was provided by a research grant from the Department of Environmental Sciences, International Center for Science and High Technology and Environmental Sciences. I would like to acknowledge Mr. M. Torkzadeh from the analytical laboratory of the International Center for Science and High Technology and Environmental Sciences. The paper is part of the M.Sc. Thesis of the first author carried out under supervision of Dr. A. Aftabi at Shahid Bahonar University of Kerman.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamzeh, M.A., Aftabi, A. & Mirzaee, M. Assessing geochemical influence of traffic and other vehicle-related activities on heavy metal contamination in urban soils of Kerman city, using a GIS-based approach. Environ Geochem Health 33, 577–594 (2011). https://doi.org/10.1007/s10653-010-9372-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-010-9372-0