Abstract
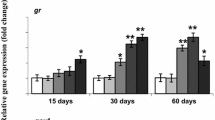
The increased use of pyrethroid insecticides raises concern for exposure to non-target aquatic species, such as Chinook salmon (Oncorhynchus tshawytscha). Cypermethrin, a type II pyrethroid, is frequently detected in surface waters and sediments at concentrations that exceed levels that induce toxicity to several invertebrate and salmonid species. To better understand the effects of cypermethrin to salmonids following dietary exposure, juvenile Chinook salmon were dietarily exposed to a 0, 200, or 2000 ng/g cypermethrin diet for a duration of 7, 14, or 21 days and assessed for body burden residues, swimming performance, lipid content, and lipid homeostatic gene expression. The average cypermethrin concentrations in fish dietarily exposed to cypermethrin for 21 days were 155.4 and 952.1 ng cypermethrin/g lipid for the 200 and 2000 ng/g pellet treatments, respectively. Increased trends of fatty acid synthase (fasn, r2 = 0.10, p < 0.05) and ATP citrate lyase (acly, r2 = 0.21, p < 0.001) mRNA expression were found in the fish livers relative to increasing cypermethrin body burden residues, though no significant changes in the mRNA expression of farnesoid X receptor or liver X receptor were observed. Furthermore, Chinook salmon dietarily exposed to cypermethrin did not have a significantly altered burst swimming performance (Umax). These results support studies that have suggested Umax may not be a sensitive endpoint when assessing the effects of certain pesticide classes, such as pyrethroids, but that dysregulation of fasn and acly expression may alter lipid homeostasis and energy metabolism in the liver of fish dietarily exposed to cypermethrin.



Similar content being viewed by others
Data availability
Data will be available on request.
References
Beggel S, Connon R, Werner I, Geist J (2011) Changes in gene transcription and whole organism responses in larval fathead minnow (Pimephales promelas) following short-term exposure to the synthetic pyrethroid bifenthrin. Aquat Toxicol 105(1–2):180–188. https://doi.org/10.1016/j.aquatox.2011.06.004
Bonansea RI, Wunderlin DA, Amé MV (2016) Behavioral swimming effects and acetylcholinesterase activity changes in Jenynsia multidentata exposed to chlorpyrifos and cypermethrin individually and in mixtures. Ecotoxicol Environ Saf 129:311–319. https://doi.org/10.1016/j.ecoenv.2016.03.043
Bonansea RI, Marino DJ, Bertrand L, Wunderlin DA, Amé MV (2017) Tissue‐specific bioconcentration and biotransformation of cypermethrin and chlorpyrifos in a native fish (Jenynsia multidentata) exposed to these insecticides singly and in mixtures. Environ Toxicol Chem 36(7):1764–1774. https://doi.org/10.1002/etc.3613
Bradbury SP, Coats JR (1989) Comparative toxicology of the pyrethroid insecticides. In: Ware GW (ed.) Reviews of environmental contamination and toxicology. Springer, New York, NY, p 133–177. https://doi.org/10.1007/978-1-4613-8850-0_4
Brett JR (1964) The respiratory metabolism and swimming performance of young sockeye salmon. J Fish Res Board Canada 21:1183–1226. https://doi.org/10.1139/f64-103
Budd R, Bondarenk S, Haver D, Kabashima J, Gan J (2007) Occurrence and bioavailability of pyrethroids in a mixed land use watershed. J Environ Qual 36:1006–1012. https://doi.org/10.2134/jeq2006.0249
Burridge L, Weis JS, Cabell F, Pizarro J, Bostick K (2010) Chemical use in salmon aquaculture: a review of current practices and possible environmental effects. Aquaculture 306:7–23. https://doi.org/10.1016/j.aquaculture.2010.05.020
Chypre M, Zaidi N, Smans K (2012) ATP-citrate lyase: a mini-review. Biochem Biophys Re Commun 422:1–4. https://doi.org/10.1016/j.bbrc.2012.04.144
Cleveland BM, Manor ML (2015) Effects of phytoestrogens on growth-related and lipogenic genes in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol C Toxicol Pharmacol 170:28–37. https://doi.org/10.1016/j.cbpc.2015.02.001
Coats JR, Symonik DM, Bradbury SP, Dyer SD, Timson LK, Atchison GJ (1989) Toxicology of synthetic pyrethroids in aquatic organisms: an overview. Environ Toxicol Chem 8:671–679. https://doi.org/10.1002/etc.5620080805
Corcellas C, Eljarrat E, Barceló D (2015) First report of pyrethroid bioaccumulation in wild river fish: a case study in Iberian river basins (Spain). Environ Int 75:110–116. https://doi.org/10.1016/j.envint.2014.11.007
Cripe GM, Goodman LR, Hansen DJ (1984) Effect of chronic exposure to EPN and to guthion on the critical swimming speed and brain acetylcholinesterase activity of Cyprinodon variegatus. Aquat Toxicol 5:255–266. https://doi.org/10.1016/0166-445X(84)90024-9
Engelking S (2018) Viability, growth, development, and performance of juvenile sockeye salmon (Oncorhynchus nerka) exposed to neonicotinoid pesticides. Dissertation, Simon Fraser University
Eni G, Ibor OR, Andem AB, Oku EE, Chukwuka AV, Adeogun AO, Arukwe A (2019) Biochemical and endocrine-disrupting effects in Clarias gariepinus exposed to the synthetic pyrethroids, cypermethrin and deltamethrin. Comp Biochem Physiol C Toxicol Pharmacol 225:108584. https://doi.org/10.1016/j.cbpc.2019.108584
Farrell AP, Johansen JA, Suarez RK (1991) Effects of exercise-training on cardiac performance and muscle enzymes in rainbow trout, Oncorhynchus mykiss. Fish Physiol Biochem 9(4):303–312. https://doi.org/10.1007/BF02265151
Farrell AP (2008) Comparisons of swimming performance in rainbow trout using constant acceleration and critical swimming speed tests. J Fish Biol 72:693–710. https://doi.org/10.1111/j.1095-8649.2007.01759.x
Fong S, Louie S, Werner I, Davis J, Connon RE (2016) Contaminant effects on California Bay–Delta species and human health. San Franc Estuary Watershed Sci 14:4. https://doi.org/10.15447/sfews.2016v14iss4art5
Gan J, Lee SJ, Liu WP, Haver DL, Kabashima JN (2005) Distribution and persistence of pyrethroids in runoff sediments. J Environ Qual 34:836–841. https://doi.org/10.2134/jeq2004.0240
Gibb AC, Dickson KA (2002) Functional morphology and biochemical indices of performance: is there a correlation between metabolic enzyme activity and swimming performance? Integr Comp Biol 42(2):199–207. https://doi.org/10.1093/icb/42.2.199
Glaser JA, Foerst DL, McKee GD, Quave SA, Budde WL (1981) Trace analyses for wastewaters. Environ Sci Tech 15(12):1426–1435. https://doi.org/10.1021/es00094a002
Goertler P, Jones K, Cordell J, Schreier B, Sommer T (2018) Effects of extreme hydrologic regimes on juvenile Chinook salmon prey resources and diet composition in a large river floodplain. Trans Am Fish Soc 147(2):287–299. https://doi.org/10.1002/tafs.10028
Goertzen MM, Driessnack MK, Janz DM, Weber LP (2011) Swimming performance and energy homeostasis in juvenile laboratory raised fathead minnow (Pimephales promelas) exposed to uranium mill effluent. Comp Biochem Physiol C Toxicol Pharmacol 4:420–426. https://doi.org/10.1016/j.cbpc.2011.07.012
Goulding AT, Shelley LK, Ross PS, Kennedy CJ (2013) Reduction in swimming performance in juvenile rainbow trout (Oncorhynchus mykiss) following sublethal exposure to pyrethroid insecticides. Comp Biochem Physiol C Toxicol Pharmacol 157:280–286. https://doi.org/10.1016/j.cbpc.2013.01.001
Hong J, Kim HY, Kim DG, Seo J, Kim KJ (2004) Rapid determination of chlorinated pesticides in fish by freezing-lipid filtration, solid-phase extraction and gas chromatography–mass spectrometry. J Chromatogr A 1038(1–2):27–35. https://doi.org/10.1016/j.chroma.2004.03.003
Hunt L, Bonetto C, Marrochi N, Scalise A, Fanelli S, Liess M, Lydy MJ, Chiu MC, Resh VH (2017) Species at Risk (SPEAR) index indicates effects of insecticides on stream invertebrate communities in soy production regions of the Argentine Pampas. Sci Total Environ 580:699–709. https://doi.org/10.1016/j.scitotenv.2016.12.016
Hunt L, Bonetto C, Resh VH, Buss DF, Fanelli S, Marrochi N, Lydy MJ (2016) Insecticide concentrations in stream sediments of soy production regions of South America. Sci Total Environ 547:114–124. https://doi.org/10.1016/j.scitotenv.2015.12.140
Jeffries KM, Komoroske LM, Truong J, Werner I, Hasenbein M, Hasenbein S et al. (2015) The transcriptome-wide effects of exposure to a pyrethroid pesticide on the Critically Endangered delta smelt Hypomesus transpacificus. Endanger Species Res 28(1):43–60. https://doi.org/10.3354/esr00679
Jin Y, Lin X, Miao W, Wang L, Wu Y, Fu Z (2015) Oral exposure of pubertal male mice to endocrine-disrupting chemicals alters fat metabolism in adult livers. Far East Entomol 30:1434–1444. https://doi.org/10.1002/tox.22013
Kumaraguru AK, Beamish FWH (1983) Bioenergetics of acclimation to permethrin (NRDC-143) by rainbow trout. Comp Biochem Physiol 75C:247–252
Li H, Cheng F, Wei Y, Lydy MJ, You J (2017) Global occurrence of pyrethroid insecticides in sediment and the associated toxicological effects on benthic invertebrates: an overview. J Hazard Mater 324:258–271. https://doi.org/10.1016/j.jhazmat.2016.10.056
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Lu Z, Gan J, Cui X, Delgado-Moreno L, Lin K (2019) Understanding the bioavailability of pyrethroids in the aquatic environment using chemical approaches. Environ Int 129:194–207. https://doi.org/10.1016/j.envint.2019.05.035
Magnuson JT, Giroux M, Cryder Z, Gan J, Schlenk D (2020a) The use of non-targeted metabolomics to assess the toxicity of bifenthrin to juvenile Chinook salmon (Oncorhynchus tshawytscha). Aquat Toxicol 224:105518. https://doi.org/10.1016/j.aquatox.2020.105518
Magnuson JT, Cryder Z, Andrzejczyk NE, Harraka G, Wolf DC, Gan J, Schlenk D (2020b) Metabolomic profiles in brains of juvenile steelhead (Oncorhychus mykiss) following bifenthrin treatment. Environ Sci Tech 54(19):12245–12253. https://doi.org/10.1021/acs.est.0c04847
Marino D, Ronco A (2005) Cypermethrin and chlorpyrifos concentration levels in surface water bodies of the Pampa Ondulada, Argentina. Bull Environ Contam Toxicol 75:820–826. https://doi.org/10.1007/s00128-005-0824-7
Maund SJ, Hamer MJ, Lane MCG, Farrelly E, Rapley JH, Goggin UM, Gentle WE (2002) Partitioning, bioavailability, and toxicity of the pyrethroid insecticide cypermethrin in sediments. Environ Toxicol Chem 21:9–15. https://doi.org/10.1002/etc.5620210102
Meador JP, Sommers FC, Ylitalo GM, Sloan CA (2006) Altered growth and related physiological responses in juvenile Chinook salmon (Oncorhynchus tshawytscha) from dietary exposure to polycyclic aromatic hydrocarbons (PAHs). Can J Fish Aquat Sci 63:2364–2376. https://doi.org/10.1139/F06-127
Meyer BN, Lam C, Moore S, Jones RL (2013) Laboratory degradation rates of 11 pyrethroids under aerobic and anaerobic conditions. J Agric Food Chem 61:4702–4708. https://doi.org/10.1021/jf400382u
Motomura H, Narahashi T (2000) Temperature dependence of pyrethroid modification of single sodium channels in rat hippocampal neurons. J Membr Biol 177(1):23–39. https://doi.org/10.1007/s002320001097
Moustafa GG, Hussein MMA (2016) Lambda cyhalothrin toxicity induces alterations in lipogenic genes and inflammatory factors in rat liver. Jpn J Vet Res 64:25–38. https://doi.org/10.14943/jjvr.64.1.25
Muir DC, Hobden BR, Servos MR (1994) Bioconcentration of pyrethroid insecticides and DDT by rainbow trout: uptake, depuration, and effect of dissolved organic carbon. Aquat Toxicol 29(3-4):223–240. https://doi.org/10.1016/0166-445X(94)90070-1
Nasuti C, Cantalamessa F, Falcioni G, Gabbianelli R (2003) Different effects of type I and type II pyrethroids on erythrocyte plasma membrane properties and enzymatic activity in rats. Toxicology 191:233–244. https://doi.org/10.1016/S0300-483X(03)00207-5
Nowell L (2003) Organochlorine pesticides and PCBs in bed sediment and whole fish from United States rivers and streams: Summary statistics: preliminary results from cycle I of the National Water Quality Assessment Program (NAWQA 1992-2001). http://ca.water.usgs.gov/pnsp/oc_doc.html. Accessed 12 Dec 2020
Olsvik PA, Larsen AK, Berntssen MHG, Goksøyr A, Karlsen OA, Yadetie F, Sanden M, Kristensen T (2019) Effects of agricultural pesticides in aquafeeds on wild fish feeding on leftover pellets near fish farms. Front Genet 10 https://doi.org/10.3389/fgene.2019.00794
Sun BQ, Wang F, Li HZ, You J (2015) Occurrence and toxicity of sediment-associated contaminants in Guangzhou College City and its adjacent areas: the relationship to urbanization. Arch Environ Contam Toxicol 68(1):124–131. https://doi.org/10.1007/s00244-014-0097-4
Tang W, Wang D, Wang J, Wu Z, Li L, Huang M, Yan D et al. (2018) Pyrethroid pesticide residues in the global environment: an overview. Chemosphere 191:990–1007. https://doi.org/10.1016/j.chemosphere.2017.10.115
Thangavel P, Sumathiral K, Karthikeyan S, Ramaswamy M (2005) Endocrine response of the freshwater teleost, Sarotherodon mossambicus (Peters) to dimecron exposure. Chemosphere 61(8):1083–1092. https://doi.org/10.1016/j.chemosphere.2005.03.045
Tierney K, Casselman M, Takeda S, Farrell T, Kennedy C (2007) The relationship between cholinesterase inhibition and two types of swimming performance in chlorpyrifos-exposed coho salmon (Oncorhynchus kisutch). Environ Toxicol Chem 26:998–1004. https://doi.org/10.1897/06-459R.1
Ullah S, Zuberi A, Alagawany M, Farag MR, Dadar M, Karthik K et al. (2018) Cypermethrin induced toxicities in fish and adverse health outcomes: Its prevention and control measure adaptation. J Environ Manage 206:863–871. https://doi.org/10.1016/j.jenvman.2017.11.076
US Environmental Protection Agency (2008) Reregistration eligibility decision for cypermethrin (revised 01/14/08). EPA, Washington, DC
Van Handel E (1985) Rapid determination of total lipids in mosquitoes. J Am Mosq Control Assoc 1(3):302–304
Vijverberg HPM, vanden Bercken J (1990) Neurotoxicological effects and the mode of action of pyrethroid insecticides. Crit Rev Toxicol 21:105–126. https://doi.org/10.3109/10408449009089875
Wang D, Weston DP, Lydy MJ (2009) Method development for the analysis of organophosphate and pyrethroid insecticides at low parts per trillion levels in water. Talanta 78(4–5):1345–1351. https://doi.org/10.1016/j.talanta.2009.02.012
Watanabe T (1982) Lipid nutrition in fish. Comp Biochem Physiol 73B:3–15
Werner I, Geist J, Okihiro M, Rosenkranz P, Hinton DE (2002) Effects of dietary exposure to the pyrethroid pesticide esfenvalerate on medaka (Oryzias latipes). Mar Environ Res 54(3–5):609–614. https://doi.org/10.1016/S0141-1136(02)00151-4
Weston DP, Holmes RW, Lydy MJ (2009) Residential runoff as a source of pyrethroid pesticides to urban creeks. Environ Pollut 157:287–294. https://doi.org/10.1016/j.envpol.2008.06.037
Weston DP, Schlenk D, Riar N, Lydy MJ, Brooks ML (2015) Effects of pyrethroid insecticides in urban runoff on Chinook salmon, steelhead trout, and their invertebrate prey. Environ Toxicol Chem 34:649–657. https://doi.org/10.1002/etc.2850
Xiang D, Chu T, Li M, Wang Q, Zhu G (2018) Effects of pyrethroid pesticide cis-bifenthrin on lipogenesis in hepatic cell line. Chemosphere 201:840–849. https://doi.org/10.1016/j.chemosphere.2018.03.009
Yang JS, Qi W, Farias-Pereira R, Choi S, Clark JM, Kim D, Park Y (2019) Permethrin and ivermectin modulate lipid metabolism in steatosis-induced HepG2 hepatocyte. Food Chem Toxicol 125:595–604. https://doi.org/10.1016/j.fct.2019.02.005
Yilmaz M, Gül A, Erbaşli K (2004) Acute toxicity of alpha-cypermethrin to guppy (Poecilia reticulata, Pallas, 1859). Chemosphere 56:381–385. https://doi.org/10.1016/j.chemosphere.2004.02.034
You J, Lydy MJ (2007) A solution for isomerization of pyrethroid insecticides in gas chromatography. J Chromatogr A 1166:181–190. https://doi.org/10.1016/j.chroma.2007.08.014
Zhang J, You J, Li H, Tyler Mehler W, Zeng EY (2018) Particle-scale understanding of cypermethrin in sediment: desorption, bioavailability, and bioaccumulation in benthic invertebrate Lumbriculus variegatus. Sci Total Environ 642:638–645. https://doi.org/10.1016/j.scitotenv.2018.06.098
Acknowledgements
We thank the anonymous reviewers for their thoughtful comments which improved the manuscript.
Funding
This research was funded through the California Department of Fish and Wildlife Proposition 1 Restoration Grant Program (#P1896015).
Author information
Authors and Affiliations
Contributions
All authors contributed to the production of the manuscript and research project.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experiments were performed in accordance with the Institutional Animal Care and Use Committee (Protocol number: 17-027) approved by Southern Illinois University IACUC. No studies with human participants are included in this research project.
Informed consent
All authors are aware of the informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Fuller, N., Magnuson, J.T., Huff Hartz, K.E. et al. Effects of dietary cypermethrin exposure on swimming performance and expression of lipid homeostatic genes in livers of juvenile Chinook salmon, Oncorhynchus tshawytscha. Ecotoxicology 30, 257–267 (2021). https://doi.org/10.1007/s10646-021-02352-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-021-02352-2