Abstract
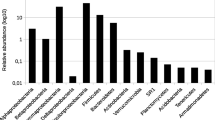
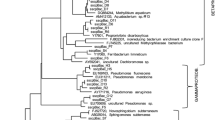
A survey of bacterial and archaeal community structure was carried out in 10 shallow tube wells in a high arsenic groundwater system located in Hetao Basin, Inner Mongolia by 16S rRNA gene based two-step nested PCR-DGGE, clone libraries and 454 pyrosequencing. 12 bacterial and 18 archaeal DGGE bands and 26–136 species-level OTUs were detected for all the samples. 299 bacterial and 283 archaeal 16S rRNA gene clones for two typical samples were identified by phylogenetic analysis. Most of the results from these different methods were consistent with the dominant bacterial populations. But the proportions of the microbial populations were mostly different and the bacterial communities in most of these samples from pyrosequencing were both more abundant and more diverse than those from the traditional methods. Even after quality filtering, pyrosequencing revealed some populations including Alishewanella, Sulfuricurvum, Arthrobacter, Sporosarcina and Algoriphagus which were not detected with traditional techniques. The most dominant bacterial populations in these samples identified as some arsenic, iron, nitrogen and sulfur reducing and oxidizing related populations including Acinetobacter, Pseudomonas, Flavobacterium, Brevundimonas, Massilia, Planococcus, and Aquabacterium and archaeal communities Nitrosophaera and Methanosaeta. Acinetobacter and Pseudomonas were distinctly abundant in most of these samples. Methanogens were found as the dominant archeal population with three methods. From the results of traditional methods, the dominant archaeal populations apparently changed from phylum Thaumarchaeota to Euryarchaeota with the arsenic concentrations increasing. But this structure dynamic change was not revealed with pyrosequencing. Our results imply that an integrated approach combining the traditional methods and next generation sequencing approaches to characterize the microbial communities in high arsenic groundwater is recommended.








Similar content being viewed by others
References
Berg M, Stengel C, Trang PTK, Viet PH, Sampson ML, Leng M, Samreth S, Fredericks D (2007) Magnitude of arsenic pollution in the Mekong and Red River Deltas-Combodia and Vietnam. Sci Total Environ 372:413–425
Bouétard A, Noirot C, Besnard A-L, Bouchez O, Choisne D, Robe E, Klopp C, Lagadic C, Coutellec M-A (2012) Pyrosequencing-based transcriptomic resources in the pond snail Lymnaea stagnalis, with a focus on genes involved in molecular response to diquat-induced stress. Ecotoxicology 21:2222–2234
Costa KC, Navarro JB, Shock EL, Zhang CL, Soukup D (2009) Microbiology and geochemistry of great boiling and mud hot springs in the United States Great Basin. Extremophiles 13:447–459
DeLong EF (1992) Archaea in coastal marine environments. Proc Natl Acad Sci USA 89:5685–5689
Deng YM (2008) Geochemical proeesses of high arsenic groundwate system at Western Hetao Basin. Dissertation, China University of Geoscience
Deng YM, Wang YX, Ma T (2009) Speciation and enrichment of arsenic in strongly reducing shallow aquifers at western Hetao Plain, northern China. Environ Geol 56:1467–1477
Dodsworth JA, Hungate BA, Hedlund BP (2011) Ammonia oxidation, denitrification and dissimilatory nitrate reduction to ammonium in two US Great Basin hot springs with abundant ammonia-oxidizing archaea. Environ Microb 13:2371–2386
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461
Fan H, Su C, Wang Y, Yao J, Zhao K, Wang G (2008) Sedimentary arsenite-oxidizing and arsenate-reducing bacteria associated with high arsenic groundwater from Shanyin, Northwestern China. J Appl Microb 105:529–539
Farooq SH, Chandrasekharam D, Abbt-Braun G, Berner Z, Norra S, Stueben D (2012) Dissolved organic carbon from the traditional jute processing technique and its potential influence on arsenic enrichment in the Bengal Delta. Appl Geochem 27:292–303
Fisher OE, Dawson AM, Polshyna G, Ann NY (2008) Transformation of inorganic and organic arsenic by Alkaliphilus oremlandii sp. nov. Strain Acad Sci 1125:230–241
Freikowski D, Winter J, Gallert C (2010) Hydrogen formation by an arsenate-reducing Pseudomonas putida, isolated from arsenic-contaminated groundwater in West Bengal, India. Appl Microbiol Biotechnol 88:1363–1371
Guo H, Tang X, Yang S, Shen Z (2008) Effect of indigenous bacteria on geochemical behavior of arsenic in aquifer sediments from the Hetao Basin, Inner Mongolia: evidence from sediment incubations. Appl Geochem 23:3267–3277
He J, Ma T, Deng Y, Yang H, Wang Y (2009) Environmental geochemistry of high arsenic groundwater at western Hetao plain, Inner Mongolia. Front Earth Sci China 3:63–72
Herbert KJ, Snow ET (2012) Modulation of arsenic-induced epidermal growth factor receptor pathway signalling by resveratrol. Chem-Biol Interact 198:38–48
Hohmann C, Morin G, Ona-Nguema G, Guigner J-M, Brown GE Jr, Kappler A (2011) Molecular-level modes of As binding to Fe(III) (oxyhydr)oxides precipitated by the anaerobic nitrate-reducing Fe(II)-oxidizing Acidovorax sp. strain BoFeN1. Geochim Cosmochim Acta 75:4699–4712
Hou W, Wang S, Dong H, Jiang H, Briggs BR, Peacock JP, Huang Q, Huang L, Wu G, Zhi X (2013) A comprehensive census of microbial diversity in hot springs of Tengchong, Yunnan Province China using 16S rRNA gene pyrosequencing. PLoS One 8(1):e53350
Islam FS, Gault AG, Boothman C, Polya DA, Charnock JM, Chatterjee D, Lloyd JR (2004) Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature 430:68–71
Jiang H, Dong H, Zhang G, Yu B, Chapman LR, Fields MW (2006) Microbial diversity in water and sediment of Lake Chaka, an athalassohaline lake in Northwestern China. Appl Environ Microbiol 72:7430–7431
Jiang Z, Li P, Wang Y, Li B, Deng Y, Wang Y (2014) Vertical distribution of bacterial populations associated with arsenic mobilization in aquifer sedimentsfrom the Hetao plain, Inner Mongolia. Environ Earth Sci 71:311–318
Kautz S, Rubin BE, Russell JA, Moreau CS (2013) Surveying the microbiome of ants: comparing 454 pyrosequencing with traditional methods to uncover bacterial diversity. Appl Environ Microbiol 79:525–534
Keshri J, Mishra A, Jha B (2013) Microbial population index and community structure in saline–alkaline soil using gene targeted metagenomics. Microbiol Res 168:165–173
Knight R, Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ (2010) PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26:266–267
Kocar BD, Borch T, Fendorf S (2010) Arsenic repartitioning during biogenic sulfidization and transformation of ferrihydrite. Geochim Cosmochim Acta 74:980–994
Li P, Wang Y, Wang Y, Liu K, Tong L (2010) Bacterial community structure and diversity during establishment of an anaerobic bioreactor to treat swine wastewater. Water Sci Technol 61:243–252
Li P, Wang Y, Jiang Z, Jiang H, Li B, Dong H, Wang Y (2013) Microbial diversity in high arsenic groundwater in Hetao Basin of Inner Mongolia, China. Geomicrobiol J 30:897–909
Liao VH, Chu YJ, Su YC, Hsiao SY, Wei CC, Liu CW, Liao CM, Shen WC, Chang FJ (2011) Arsenite-oxidizing and arsenate-reducing bacteria associated with arsenic-rich groundwater in Taiwan. J Contam Hydrol 123:20–29
Malasarn D, Saltikov CW, Campbell KM, Santini JM, Hering JG, Newman DK (2004) arrA is a reliable marker for As(V) respiration. Science 306:455
Marchesi JR, Sato T, Weightman AJ, Martin TA, Fry JC, Hiom SJ, Wade WG (1998) Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol 64:795–799
Mukherjee A, Bhattacharya P, Shi F (2009) Chemical evolution in the high arsenic groundwater of the Huhhot basin(Inner Mongolia, PR China) and its difference from the western Bengal basin (India). Appl Geochem 24:1835–1851
Neidhardt H, Norra S, Tang X, Guo H, Stüben D (2012) Impact of irrigation with high arsenic burdened groundwater on the soil–plant system: results from a case study in the Inner Mongolia, China. Environ Pollut 163:8–13
Nicol GW, Glover LA, Prosser JI (2003) The impact of grassland management on archaeal community structure in upland pasture rhizosphere soil. Environ Microbiol 5:152–162
Nordstrom DK (2002) Worldwide occurrences of arsenic in ground water. Science 296:2143–2145
O’Sullivan LA, Webster G, Fry JC, Parkes RJ, Weightman AJ (2008) Modified linker-PCR primers facilitate complete sequencing of DGGE DNA fragments. J Microbiol Methods 75:579–581
Pe′rez-Jime′nez JR, DeFraia C, Young LY (2005) Arsenate respiratory reductase gene (arrA) for Desulfosporosinus sp. strain Y5. Biochem Biophys Res Commun 338:825–829
Pinto AJ, Raskin L (2012) PCR biases distort bacterial and archaeal community structure in pyrosequencing datasets. PLoS One 7:e43093
Polya D, Charlet L (2009) Environmental science: Rising arsenic risk? Nat Geosci 2:383–384
Riemer J, Hoepken HH, Czerwinska H, Robinson SR, Dringen R (2004) Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal Biochem 331:370–375
Rodríguez-Lado L, Sun G, Berg M, Zhang Q, Xue H, Zheng Q, Johnson CA (2013) Groundwater arsenic contamination throughout China. Science 341:866–868
Schloss PD, Handelsman J (2004) Status of the microbial census. Microbiol Mol Biol Rev 68:686–691
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Micobiol 75:7537
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Smith AH, Lopipero PA, Bates MN, Steinmaus CM (2002) Arsenic epidemiology and drinking water standards. Science 296:2145–2146
Song ZQ, Chen JQ, Jiang HC, Zhou EM (2010) Diversity of Crenarchaeota in terrestrial hot springs in Tengchong, China. Extremophiles 14:287–296
Srivastava D, Madamwar D, Subramanian RB (2010) Pentavalent arsenate reductase activity in cytosolic fractions of Pseudomonas sp., isolated from arsenic-contaminated sites of Tezpur, Assam. Appl Biochem Biotechnol 162:3766–3779
Sundberg C, Al-Soud WA, Larsson M, Alm E, Yekta SS, Svensson BH, Sørensen SJ, Karlsson A (2013) 454 pyrosequencing analyses of bacterial and archaeal richness in 21 full-scale biogas digesters. FEMS Microbiol Ecol 85:612–626
Sutton NB, van der Kraan GM, van Loosdrecht MCM, Muyzer G, Bruining J, Schotting RJ (2009) Characterization of geochemical constituents and bacterial populations associated with As mobilization in deep and shallow tube wells in Bangladesh. Water Res 43:1720–1730
Vick TJ, Dodsworth JA, Costa KC, Shock EL, Hedlund BP (2010) Microbiology and geochemistry of Little Hot Creek, a hot spring environment in the Long Valley Caldera. Geobiology 8:140–154
Webster G, Parkes RJ, Cragg BA, Newberry CJ, Weightman AJ, Fry JC (2006) Prokaryotic community composition and biogeochemical processes in deep subseafloor sediments from the Peru Margin. FEMS Microbiol Ecol 58:65–85
Zhou J, Wu L, Deng Y, Zhi X, Jiang Y-H, Tu Q, Xie J, Van Nostrand JD, He Z, Yang Y (2011) Reproducibility and quantitation of amplicon sequencing-based detection. ISME J 5:1303–1313
Acknowledgements
This research was financially supported by National Natural Science Foundation of China (Grant No. 41372348, 41120124003), National Science Foundation for Post-doctoral Scientists of China (Grant No. 2012M521491, 2013T60757) and the Fundamental Research Funds for the Central Universities, China University of Geosciences (No. CUG140505, GBL11204)
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, P., Jiang, D., Li, B. et al. Comparative survey of bacterial and archaeal communities in high arsenic shallow aquifers using 454 pyrosequencing and traditional methods. Ecotoxicology 23, 1878–1889 (2014). https://doi.org/10.1007/s10646-014-1316-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-014-1316-5