Abstract
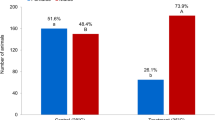
Hypoxia caused by eutrophication is amongst the most pressing global problems in aquatic systems. Notably, more than 400 “dead zones” have been identified worldwide, resulting in large scale collapse of fisheries and major changes in the structure and trophodynamics. Recent studies further discovered that hypoxia can also disrupt sex hormone metabolism and alter the sexual differentiation of fish, resulting in male biased F1 generations and therefore posing a threat to the sustainability of natural populations. However, it is not known whether, and if so how, hypoxia can also change the sex ratio in vertebrates that have sex-determining XX/XY chromosomes. Using the Japanese medaka (Oryzias latipes) as a model, we demonstrate, for the first time, that hypoxia can turn genotypic female fish with XX chromosomes into phenotypic males. Over half of the XX females exposed to hypoxia exhibit male secondary sexual characteristics and develop testis instead of ovary. We further revealed that hypoxia can: (a) down-regulate the vasa gene, which controls proliferation of primordial germ cells and gonadal sex differentiation into ovary, and (b) up-regulate the DMY gene which resides at the sex-determining locus of the Y chromosome, and direct testis differentiation. This is the first report that hypoxia can directly act on genes that regulate sex determination and differentiation, thereby turning genotypic females into phenotypic males and leading to a male-dominant F1 population.




Similar content being viewed by others
References
Alderdice DF, Wickett WP, Brett JR (1958) Some effects of temporary exposure to low dissolved oxygen levels on pacific salmon eggs. J Fish Res Board Can 15:229–250
Ankoma-Sey V, Wang Y, Dai Z (2000) Hypoxic stimulation of vascular endothelial growth factor expression in activated rat hepatic stellate cells. Hepatology 31:141–148
Baroiller JF, D’Cotta H, Saillant E (2009) Environmental effects on fish sex determination and differentiation. Sex Dev 3:118–135
Blázquez M, González A, Mylonas CC, Piferrer F (2011) Cloning and sequence analysis of a vasa homolog in the European sea bass (Dicentrarchus labrax): tissue distribution and mRNA expression levels during early development and sex differentiation. Gen Comp Endocrinol 170:322–333
Boswell MG, Wells MC, Kirk LM, Ju Z, Zhang Z, Booth RE, Walter RB (2009) Comparison of gene expression responses to hypoxia in viviparous (Xiphophorus) and oviparous (Oryzias) fishes using a medaka microarray. Comp Biochem Physiol C 149:258–265
Braat AK, Zandbergen T, Van de Water S, Goos HJT, Zivkovic D (1999) Characterization of zebrafish primordial germ cells: morphology and early distribution of vasa RNA. Dev Dyn 216:153–167
Burggren W (1999) Genetic, environmental and maternal influences on embryonic cardiac rhythms. Comp Biochem Physiol A 124:423–427
Czerkies P, Brzuzan P, Kordalski K, Luczynski M (2001) Critical partial pressures of oxygen causing precocious hatching in Coregonus lavaretus and C. albula embryos. Aquaculture 196:151–158
Devlin RH, Nagahama Y (2002) Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208:191–364
Diaz RJ, Rosenberg R (2008) Spreading dead zones and consequences for marine ecosystems. Science 321:926–929
Diaz RJ, Rosenberg R (2011) Introduction to environmental and economic consequences of hypoxia. Int J Water Resour D 27:71–82
Geist DR, Abernethy CS, Hand KD, Cullinan VI, Chandler JA, Groves PA (2006) Survival, development, and growth of fall Chinook salmon embryos, alevins, and fry exposed to variable thermal and dissolved oxygen regimes. Trans Am Fish Soc 135:1462–1477
Godwin J, Luckenbach JA, Borski RJ (2003) Ecology meets endocrinology: environmental sex determination in fishes. Evol Dev 5:40–49
Gray JS, Wu RSS, Or YY (2002) Effects of hypoxia and organic enrichment on the coastal marine environment. Mar Ecol Prog Ser 238:249–279
Grim KC, Wolfe M, Hawkins W, Johnson R, Wolf J (2007) Intersex in Japanese medaka (Oryzias latipes) used as negative controls in toxicologic bioassays: a review of 54 cases from 41 studies. Environ Toxicol Chem 26:1636–1643
Iwamatsu T (2004) Stages of normal development in the medaka Oryzias latipes. Mech Dev 121:605–618
Jewell UR, Kvietikova I, Scheid A, Bauer C, Wenger RH, Gassmann M (2001) Induction of HIF-1α in response to hypoxia is instantaneous. FASEB J 15:1312–1314
Keckeis H, Bauer-Nemeschkal E, Kamler E (1996) Effects of reduced oxygen level on the mortality and hatching rate of Chondrostoma nasus embryos. J Fish Biol 49:430–440
Kobayashi T, Kajiura-Kobayashi H, Nagahama Y (2000) Differential expression of vasa homologue gene in the germ cells during oogenesis and spermatogenesis in a teleost fish, tilapia, Oreochromis niloticus. Mech Dev 99:139–142
Kobayashi T, Matsuda M, Kajiura-Kobayashi H, Suzuki A, Saito N, Nakamoto M, Shibata N, Nagahama Y (2004) Two DM domain genes, DMY and DMRT1, involved in testicular differentiation and development in the medaka, Oryzias latipes. Dev Dyn 231:518–526
Kurauchi K, Nakaguchi Y, Tsutsumi M, Hori H, Kurihara R, Hashimoto S, Ohnuma R, Yamamoto Y, Matsuoka S, Kawai S, Hirata T, Kinoshita M (2005) In vivo visual reporter system for detection of estrogen-like substances by transgenic medaka. Environ Sci Technol 39:2762–2768
Landry CA, Steele SL, Manning S, Cheek AO (2007) Long term hypoxia suppresses reproductive capacity in the estuarine fish, Fundulus grandis. Comp Biochem Physiol A 148:317–323
Liu CC, Chiu JMY, Li L, Shin PKS, Cheung SG (2011) Physiological responses of two sublittoral nassariid gastropods to hypoxia. Mar Ecol Prog Ser 429:75–85
Lo KH, Hui MNY, Yu RMK, Wu RSS, Cheng SH (2011) Hypoxia impairs primordial germ cell migration in Zebrafish (Danio rerio) embryos. PLoS One 6:e24540
Lorke DE, Yew DT (2005) Worldwide decline of sturgeons. Science 310:1427–1429
Lu XY, Yu RMK, Murphy MB, Lau K, Wu RSS (2014) Hypoxia disrupts gene modulation along the brain–pituitary–gonad (BPG)–liver axis. Ecotoxicol Environ Saf 102:70–78
Martinovic D, Villeneuve DL, Kahl MD, Blake LS, Brodin JD, Ankley GT (2009) Hypoxia alters gene expression in the gonads of zebrafish (Danio rerio). Aquat Toxicol 95:258–272
Matsuda M (2005) Sex determination in the teleost medaka, Oryzias latipes. Annu Rev Genet 39:293–307
Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, Morrey CE, Shibata N, Asakawa S, Shimizu N, Hori H, Hamaguchi S, Sakaizumi M (2002) DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 417:559–563
Nanda I, Kondo M, Hornung U, Asakawa S, Winkler C, Shimizu A, Shan Z, Haaf T, Shimizu N, Shima A, Schmid M, Schartl M (2002) A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc Natl Acad Sci USA 99:11778–11783
Oehlers LP, Perez AN, Walter RB (2007) Detection of hypoxia-related proteins in medaka (Oryzias latipes) brain tissue by difference gel electrophoresis and de novo sequencing of 4-sulfophenyl isothiocyanate-derivatized peptides by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Comp Biochem Physiol C 145:120–133
Paul-Prasanth B, Matsuda M, Lau EL, Suzuki A, Sakai F, Kobayashi T, Nagahama Y (2006) Knock-down of DMY initiates female pathway in the genetic male medaka, Oryzias latipes. Biochem Biophys Res Commun 351:815–819
Pincetich CA, Viant MR, Hinton DE, Tjeerdema RS (2005) Metabolic changes in Japanese medaka (Oryzias latipes) during embryogenesis and hypoxia as determined by in vivo 31P NMR. Comp Biochem Physiol C 140:103–113
Roussel JM (2007) Carry-over effects in brown trout (Salmo trutta): hypoxia on embryos impairs predator avoidance by alevins in experimental channels. Can J Fish Aquat Sci 64:786–792
Satoh N, Egami N (1972) Sex differentiation of germ cells in the teleost, Oryzias latipes, during normal embryonic development. J Embryol Exp Morphol 28:385–395
Shang EHH, Wu RSS (2004) Aquatic hypoxia is a teratogen and affects fish embryonic development. Environ Sci Technol 38:4763–4767
Shang EHH, Yu RMK, Wu RSS (2006) Hypoxia affects sex differentiation and development, leading to a male-dominated population in Zebrafish (Danio rerio). Environ Sci Technol 40:3118–3122
Shima A, Mitani H (2004) Medaka as a research organism: past, present and future. Mech Dev 121:599–604
Shinomiya A, Tanaka M, Kobayashi T, Nagahama Y, Hamaguchi S (2000) The vasa-like gene, olvas, identifies the migration path of primordial germ cells during embryonic body formation stage in the medaka, Oryzias latipes. Dev Growth Differ 42:317–326
Shinomiya A, Otake H, Togashi K, Hamaguchi S, Sakaizumi M (2004) Field survey of sex-reversals in the medaka, Oryzias latipes: genotypic sexing of wild populations. Zool Sci 21:613–619
STAP (2011) Hypoxia and nutrient reduction in the coastal zone: advice for prevention, remediation and research. A STAP Advisory Document. Global Environment Facility, Washington, DC
Taglialatela R, Della Corte F (1997) Human and recombinant erythropoietin stimulate erythropoiesis in the goldfish Carassius auratus. Eur J Histochem 41:301–304
Thomas P, Rahman MS (2011) Extensive reproductive disruption, ovarian masculinization and aromatase suppression in Atlantic croaker in the northern Gulf of Mexico hypoxic zone. Proc R Soc B 279:28–38
Thomas P, Rahman MS, Kummer JA, Lawson S (2006) Reproductive endocrine dysfunction in Atlantic croaker exposed to hypoxia. Mar Environ Res 62(Suppl. 1):S249–S252
Thomas P, Rahman MS, Khan IA, Kummer JA (2007) Widespread endocrine disruption and reproductive impairment in an estuarine fish population exposed to seasonal hypoxia. Proc R Soc B 274:2693–2702
Wiener CM, Booth G, Semenza GL (1996) In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem Biophys Res Commun 225:485–488
Wu RSS (2002) Hypoxia: from molecular responses to ecosystem responses. Mar Pollut Bull 45:35–45
Wu RSS (2009) Chapter 3 effects of hypoxia on fish reproduction and development. In: Richards JG, Farrell AP, Brauner CJ (eds) Fish physiology, vol 27. Academic Press, Amsterdam, pp 79–141
Wu RSS, Zhou BS, Randall DJ, Woo NYS, Lam PKS (2003) Aquatic hypoxia is an endocrine disruptor and impairs fish reproduction. Environ Sci Technol 37:1137–1141
Yu R, Chen E, Kong R, Ng P, Mok H, Au D (2006) Hypoxia induces telomerase reverse transcriptase (TERT) gene expression in non-tumor fish tissues in vivo: the marine medaka (Oryzias melastigma) model. BMC Mol Biol 7:1–12
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, Englewood Cliffs
Zhang Z, Wu RSS, Mok HOL, Wang Y, Poon WWL, Cheng SH, Kong RYC (2003) Isolation, characterization and expression analysis of a hypoxia-responsive glucose transporter gene from the grass carp, Ctenopharyngodon idellus. Eur J Biochem 270:3010–3017
Zhang Z, Ju Z, Wells MC, Walter RB (2009) Genomic approaches in the identification of hypoxia biomarkers in model fish species. J Exp Mar Biol Ecol 381(Suppl.):S180–S187
Zhang Z, Wells MC, Boswell MG, Beldorth I, Kirk LM, Wang Y, Wang S, Savage M, Walter RB, Booth RE (2012) Identification of robust hypoxia biomarker candidates from fin of medaka (Oryzias latipes). Comp Biochem Physiol C 155:11–17
Acknowledgments
The work described in this paper was supported by a Grant from the University Grants Committee of the Hong Kong Special Administrative Region, China (AoE/P-04/04) and a postgraduate student granted to Catis Cheung by the City University of Hong Kong.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheung, C.H.Y., Chiu, J.M.Y. & Wu, R.S.S. Hypoxia turns genotypic female medaka fish into phenotypic males. Ecotoxicology 23, 1260–1269 (2014). https://doi.org/10.1007/s10646-014-1269-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-014-1269-8