Abstract
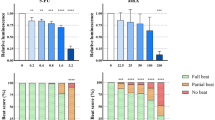
Novel methods that predict the sensitivity of avian embryos to the toxic effects of dioxin-like compounds (DLCs) using either (1) knowledge of the identity of amino acids at key sites within the ligand binding domain of aryl hydrocarbon receptor 1 (AHR1) or (2) a luciferase reporter gene assay that measures AHR1 activation were recently reported. Results from both methods predict that European starling (Sturnus vulgaris) and domestic chicken (Gallus gallus domesticus) embryos have similar sensitivity to the biochemical and toxic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), 2,3,4,7,8-pentachlorodibenzofuran (PeCDF) and 2,3,7,8-tetrachlorodibenzofuran (TCDF). Chicken embryos are highly sensitive to DLC toxicity, and the prediction that starlings are equally sensitive is surprising given their widespread distribution and large population size. In an attempt to learn more about starling sensitivity to DLCs, we determined concentration-dependent effects of TCDD, PeCDF and TCDF on cytochrome P4501A4 and 1A5 (CYP1A4 and 1A5) mRNA levels in primary cultures of hepatocytes prepared from embryonic European starlings. It has been demonstrated that the sensitivity of avian hepatocytes to CYP1A4/5 induction is well correlated with LD50 values of DLCs for several avian species. The results of the present study indicate that European starling hepatocytes are indeed as sensitive as chicken hepatocytes to CYP1A4/5 induction after exposure to TCDD. However, starling hepatocytes are less sensitive than chicken hepatocytes to CYP1A4/5 induction by PeCDF and TCDF.


Similar content being viewed by others
References
Arenal CA, Halbrook RS, Woodruff M (2004) European starling (Sturnus vulgaris): avian model and monitor of polychlorinated biphenyl contamination at a superfund site in southern Illinois, USA. Environ Toxicol Chem 23:93–104
Bastien LJ, Kennedy SW, Lorenzen A (1997) Ethoxyresorufm O-deethylase (EROD) induction by halogenated aromatic hydrocarbons (HAHS) in chicken embryo hepatocyte cultures: time-dependent effects on the dose-response curves. Organohal Comp 34:215–220
Brunstrom B (1988) Sensitivity of embryos from duck, goose, herring gull, and various chicken breeds to 3,3′,4,4′-tetrachlorobiphenyl. Poult Sci 67:52–57
Brunstrom B, Broman D, Naf C (1990) Embryotoxicity of polycyclic aromatic hydrocarbons (PAHs) in three domestic avian species, and of PAHs and coplanar polychlorinated biphenyls (PCBs) in the common eider. Environ Pollut 67:133–143
Cohen-Barnhouse AM, Zwiernik MJ, Link JE, Fitzgerald SD, Kennedy SW, Herve JC, Giesy JP, Wiseman S, Yang Y, Jones PD, Wan Y, Collins B, Newsted JL, Kay D, Bursian SJ (2011) Sensitivity of Japanese quail (Coturnix japonica), common pheasant (Phasianus colchicus) and white leghorn chicken (Gallus gallus domesticus) embryos to in ovo exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), 2,3,4,7,8- pentachlorodibenzofuran (PeCDF) and 2,3,7,8-tetrachlorodibenzofuran (TCDF). Toxicol Sci 119:93–103
Elliott JE, Wilson LK, Langelier KW, Norstrom RJ (1996) Bald eagle mortality and chlorinated hydrocarbon contaminants in livers from British Columbia, Canada, 1989–1994. Environ Pollut 94:9–18
Farmahin R, Wu D, Crump D, Herve JC, Jones SP, Hahn ME, Karchner SI, Giesy JP, Bursian SJ, Zwiernik MJ, Kennedy SW (2012) Sequence and in vitro function of chicken, ring-necked pheasant and Japanese quail AHR1 predict in vivo sensitivity to dioxins. Environ Sci Technol 46:2967–2975
Farmahin R, Manning Gillian E, Crump Doug, Dongmei Wu, Mundy Lukas, Jones Stephanie P, Hahn MarkE, Karchner sibel I, Giesy John, Bursian Steven J, Zwiernik Matthew J, Kennedy Sean W (2013) Amino acid sequence of the ligand binding domain of the aryl hydrocarbon receptor 1 (AHR1) predicts sensitivity of wild birds to effects of dioxin-like compounds. Toxicol Sci 131:139–152
Feare C (1984) The Starling. Oxford University Press, Oxford
Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ (1996) Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol 140:173–179
Giesy JP, Ludwig JP, Tillitt DE (1994) Deformities in birds of the Great Lakes region assigning causality. Environ Sci Technol 28:128A–135A
Gilbertson M, Kubiak T, Ludwig J, Fox G (1991) Great Lakes embryo mortality, edema, and deformities syndrome (GLEMEDS) in colonial fish-eating birds: similarity to chick-edema disease. J Toxicol Environ Health 33:455–520
Hahn ME (2002) Aryl hydrocarbon receptors: diversity and evolution. Chem Biol Interact 141:131–160
Halbrook RS, Arenal CA (2003) Field studies using European starlings to establish causality between PCB exposure and reproductive effects. Hum Ecol Risk Assess 9:121–136
Head JA, Kennedy SW (2007) Same-sample analysis of ethoxyresorufin-O-deethylase activity and cytochrome P4501A mRNA abundance in chicken embryo hepatocytes. Anal Biochem 360:294–302
Head JA, Kennedy SW (2010) Correlation between an in vitro and an in vivo measure of dioxin sensitivity in birds. Ecotoxicology 19:377–382
Head JA, Hahn ME, Kennedy SW (2008) Key amino acids in the aryl hydrocarbon receptor predict dioxin sensitivity in avian species. Environ Sci Technol 42:7535–7541
Herve JC, Crump D, Giesy JP, Zwiernik MJ, Bursian SJ, Kennedy SW (2010a) Ethoxyresorufin O-deethylase induction by TCDD, PeCDF and TCDF in ring-necked pheasant and Japanese quail hepatocytes: time-dependent effects on concentration-response curves. Toxicol In Vitro 24:1301–1305
Herve JC, Crump D, Jones SP, Mundy LJ, Giesy JP, Zwiernik MJ, Bursian SJ, Jones PD, Wiseman SB, Wan Y, Kennedy SW (2010b) Cytochrome P4501A induction by 2,3,7,8-tetrachlorodibenzo-p-dioxin and two chlorinated dibenzofurans in primary hepatocyte cultures of three avian species. Toxicol Sci 113:380–391
Herve JC, Crump DL, McLaren KK, Giesy JP, Zwiernik MJ, Bursian SJ, Kennedy SW (2010c) 2,3,4,7,8-pentachlorodibenzofuran is a more potent cytochrome P4501A inducer than 2,3,7,8-tetrachlorodibenzo-p-dioxin in herring gull hepatocyte cultures. Environ Toxicol Chem 29:2088–2095
Hoffman DJ, Rattner BA, Sileo L, Docherty D, Kubiak TJ (1987) Embryotoxicity, teratogenicity, and aryl hydrocarbon hydroxylase activity in Forster’s terns on Green Bay, Lake Michigan. Environ Res 42:176–184
Karchner SI, Franks DG, Kennedy SW, Hahn ME (2006) The molecular basis for differential dioxin sensitivity in birds: role of the aryl hydrocarbon receptor. Proc Natl Acad Sci USA 103:6252–6257
Kennedy SW, Jones SP, Bastien LJ (1995) Efficient analysis of cytochrome P4501A catalytic activity, porphyrins, and total proteins in chicken embryo hepatocyte cultures with a fluorescence plate reader. Anal Biochem 226:362–370
Kennedy SW, Lorenzen A, Jones SP, Hahn ME, Stegeman JJ (1996) Cytochrome P4501A induction in avian hepatocyte cultures: a promising approach for predicting the sensitivity of avian species to toxic effects of halogenated aromatic hydrocarbons. Toxicol Appl Pharmacol 141:214–230
Kennedy SW, Fox GA, Trudeau S, Bastien LJ, Jones SP (1998) Highly carboxylated porphyrin concentration: a biochemical marker of PCB exposure in herring gulls. Mar Environ Res 46:65–69
Kennedy SW, Jones SP, Elliott JE (2003) Sensitivity of bald eagle (Haliaeetus leucocephalus) hepatocyte cultures to induction of cytochrome P4501A by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Ecotoxicology 12:163–170
Kumar KS, Kannan K, Giesy JP, Masunaga S (2002) Distribution and elimination of polychlorinated dibenzo-p-dioxins, dibenzofurans, biphenyls, and p,p’-DDE in tissues of bald eagles from the Upper Peninsula of Michigan. Environ Sci Technol 36:2789–2796
Lorenzen A, Shutt LJ, Kennedy SW (1997) Sensitivity of common tern (Sterna hirundo) embryo hepatocyte cultures to CYP1A induction and porphyrin accumulation by halogenated aromatic hydrocarbons and common tern egg extracts. Arch Environ Contam Toxicol 32:126–134
Manning GE, Farmahin R, Crump D, Jones SP, Klein J, Konstantinov A, Potter D, Kennedy SW (2012) A luciferase reporter gene assay and aryl hydrocarbon receptor 1 genotype predict the LD(50) of polychlorinated biphenyls in avian species. Toxicol Appl Pharmacol 263:390–401
Manning GE, Mundy LJ, Crump D, Jones SP, Chiu S, Klein J, Konstantinov A, Potter D, Kennedy SW (2013) Cytochrome P4501A induction in avian hepatocyte cultures exposed to polychlorinated biphenyls: comparisons with AHR1-mediated reporter gene activity and in ovo toxicity. Toxicol Appl Pharmacol 266:38–47
Mimura J, Yamashita K, Nakamura K, Morita M, Takagi TN, Nakao K, Ema M, Sogawa K, Yasuda M, Katsuki M, Fujii-Kuriyama Y (1997) Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells 2:645–654
Mundy LJ, Jones SP, Crump D, Herve JC, Konstantinov A, Utley F, Potter D, Kennedy SW (2010) Highly purified hexachlorobenzene induces cytochrome P4501A in primary cultures of chicken embryo hepatocytes. Toxicol Appl Pharmacol 248:185–193
Mundy LJ, Crump D, Jones SP, Konstantinov A, Utley F, Potter D, Kennedy SW (2012) Induction of cytochrome P4501A by highly purified hexachlorobenzene in primary cultures of ring-necked pheasant and Japanese quail embryo hepatocytes. Comp Biochem Physiol C: Toxicol Pharmacol 155:498–505
Nosek JA, Craven SR, Karasov WH, Peterson RE (1993) 2,3,7,8-Tetrachlorodibenzo-p-dioxin in terrestrial environments—implications for resource-management. Wildlife Soc Bull 21:179–187
Peterson RE, Theobald HM, Kimmel GL (1993) Developmental and reproductive toxicity of dioxins and related compounds: cross-species comparisons. Crit Rev Toxicol 23:283–335
Ricklefs RE, Smeraski CA (1983) Variation in incubation period within a population of the European starling. Auk 100:926–931
Sanderson JT, Kennedy SW, Giesy JP (1998) In vitro induction of ethoxyresorufin-o-deethylase and porphyrins by halogenated aromatic hydrocarbons in avian primary hepatocytes. Environ Toxicol Chem 17:2006–2018
Schmidt JV, Carver LA, Bradfield CA (1993) Molecular characterization of the murine ahr gene—organization, promoter analysis, and chromosomal assignment. J Biol Chem 268:22203–22209
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Stickel WH, Stickel LF, Dyrland RA, Hughes DL (1984) Aroclor-1254 residues in birds—lethal levels and loss rates. Arch Environ Contam Toxicol 13:7–13
U.S.EPA (1994). Method 1613: tetra- through octa-chlorinated dioxins and furans by isotope dilution HRGC/HRMS. US Environmental Protection Agency Office of Water
Van den Steen E, Covaci A, Jaspers VL, Dauwe T, Voorspoels S, Eens M, Pinxten R (2007) Experimental evaluation of the usefulness of feathers as a non-destructive biomonitor for polychlorinated biphenyls (PCBs) using silastic implants as a novel method of exposure. Environ Int 33:257–264
Villeneuve DL, Blankenship AL, Giesy JP (2000) Derivation and application of relative potency estimates based on in vitro bioassay results. Environ Toxicol Chem 19:2835–2843
Walker MK, Catron TF (2000) Characterization of cardiotoxicity induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin and related chemicals during early chick embryo development. Toxicol Appl Pharmacol 167:210–221
Acknowledgments
We thank Pamela Martin, Glenn Barrett and Kimberly O’Hare for their leadership and contributions to all field work. This Project was funded by Environment Canada’s Ecotoxicology and Wildlife Health Division and by a University of Ottawa scholarship to Reza Farmahin.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farmahin, R., Crump, D., Jones, S.P. et al. Cytochrome P4501A induction in primary cultures of embryonic European starling hepatocytes exposed to TCDD, PeCDF and TCDF. Ecotoxicology 22, 731–739 (2013). https://doi.org/10.1007/s10646-013-1065-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-013-1065-x