Abstract
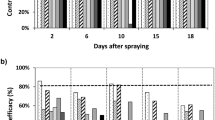
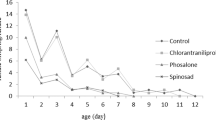
Trichogramma pretiosum Riley and T. brassicae Bezdenko are common egg parasitoids of many lepidopteran pest species damaging vegetable, but their effectiveness can be severely curtailed by insecticide applications. To identify insecticides that are potentially compatible with these parasitoid species, the effects of indoxacarb and spinosad were bioassayed in the laboratory. The bioassays included exposure of adults to various aged residues on glass and cabbage leaf surfaces at different intervals after application, and direct spray on host eggs for effects on parasitism and development and mortality of parasitoid eggs, young and old larvae and pupae. The results showed that the glass- and leaf-surface residues of indoxacarb were harmless to both parasitoid species, whereas those of spinosad were moderately harmful to harmful to both parasitoid species depending on the rates used. The use of indoxacarb on host eggs did not affect significantly parasitism by both parasitoid species, whereas the higher rates of spinosad reduced parasitism. However, both insecticides did not affect immature development and adult emergence. Results from direct spray of host eggs with various immature stages inside showed that indoxacarb did not have significant effects on the egg, young and old larval stages and the pupal stage; whereas the high rates of spinosad when applied at the older larval and pupal stages significantly reduced adult emergence for both parasitoid species. Therefore, application of spinosad in an agro-ecosystem where Trichogramma are dominant should be carefully evaluated or avoided.




Similar content being viewed by others
References
Biondi A, Desneux N, Siscaro G, Zappalà L (2012) Using organic-certified rather than synthetic pesticides may not be safer for biological control agents: selectivity and side effects of 14 pesticides on the predator Orius laevigatus. Chemosphere 87:803–812
Bostanian NJ, Akalach M (2004) The contact toxicity of indoxacarb and five other insecticides to Orius insidiosus (Hemiptera: Anthocoridae) and Aphidius colemani (Hymenoptera: Braconidae), beneficials used in the greenhouse industry. Pest Manag Sci 60:1231–1236
Bull DL, House VC (1983) Effects of different insecticides on parasitism of host eggs by Trichogramma pretiosum Riley. Southwest Entomol 8:46–53
Cartwright B, Edelson JV, Chambers C (1987) Composite action thresholds for the control of lepidopterous pests on fresh-market cabbage in the Lower Rio Grande Valley of Texas. J Econ Entomol 80:175–181
Cônsoli FL, Kitajima EW, Parra JRP (1999) Ultrastructure of the natural and factitious host eggs of Trichogramma galloi Zucchi and Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae). Int J Insect Morphol Embryol 28:211–229
Desneux N, Decourtye A, Delpuech JM (2007) The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol 52:81–106
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research, 2nd edn. John Wiley, New York
Gradish AE, Scott-Dupree CD, Shipp L, Harris CR, Ferguson G (2011) Effect of reduced risk pesticides on greenhouse vegetable arthropod biological control agents. Pest Manag Sci 67:82–86
Harder HH, Riley SL, McCann SF, Irving SN (1996) A novel broad-spectrum, environmentally soft, insect control compound. In: Brighton Conference, Brighton, DPX-M, p. 62: 1996
Haseeb M, Liu T-X, Jones WA (2004) Effects of selected insecticides on Cotesia plutellae endoparasitoid of Plutella xylostella. Biocontrol 49:33–46
Haseeb M, Amano H, Liu T-X (2005) Effects of selected insecticides on Diadegma semiclausum (Hymenoptera: Ichneumonidae) and Oomyzus sokolowskii (Hymenoptera: Eulophidae), parasitoids of Plutella xylostella (Lepidoptera: Plutellidae). Insect Sci 12:163–170
Hassan SA (1994) Strategies to select Trichogramma species for use in biological control. In: Wajnberg E, Hassan SA (eds) Biological Control With Egg Parasitoids. CAB International, Oxon, pp 55–71
Hewa-Kapuge S, McDougall S, Hoffmann AA (2003) Effects of methoxyfenozide, indoxacarb, and other insecticides on the beneficial egg parasitoid Trichogramma nr brassicae (Hymenoptera: Trichogrammatidae) under laboratory and field conditions. J Econ Entomol 96:1083–1090
Jepson PC (1989) The temporal and spatial dynamics of pesticide side-effects on non-target invertebrates. In: Jepson PC (ed) Pesticides and non-target invertebrates. Intercept, Wimborne, pp 95–128
Kareiva P (1990) The spatial dimension in pest-enemy interactions. In: Mackauer M, Ehler LE, Roland J (eds) Critical issues in biological control. Intercept, Andover, pp 213–227
Li L-Y (1994) Worldwide use of Trichogramma for biological control on different crops: a survey. In: Wajnberg E, Hassan SA (eds) Biological control with egg parasitoids. CAB International, Oxon, pp 37–53
Liu T-X, Sparks AN Jr (1999) Efficacies of some selected insecticides on cabbage looper and diamondback moth on cabbage in south Texas. Subtrop Plant Sci 51:54–58
Liu T-X, Stansly PA (1995) Deposition and bioassay of insecticides applied by leaf dip and spray tower against Bemisia argentifolii (Homoptera: Aleyrodidae). Pesticide Sci 44:317–322
Liu T-X, Sparks AN Jr, Chen W, Liang GM, Brister C (2002) Toxicity, persistence and efficacy of indoxacarb on cabbage looper (Lepidoptera: Noctuidae) on cabbage. J Econ Entomol 95:360–367
Liu T-X, Sparks AN Jr, Chen W (2003) Toxicity, persistence and efficacy of indoxacarb on Plutella xylostella (Lepidoptera: Plutellidae) in cabbage. Intern J Pest Manag 49:235–241
Liu T-X, Zhang Y-M, Peng L-N, Rojas P, Trumble JT (2012) Risk assessment of selected insecticides on Tamarixia triozae (Hymenoptera: Eulophidae), a parasitoid of Bactericera cockerelli (Hemiptera: Trizoidae). J Econ Entomol 105:490–496
Magaro JJ, Edelson JV (1990) Diamondback moth (Lepidoptera: Plutellidae) in south Texas: a technique for resistance monitoring in the field. J Econ Entomol 83:1201–1206
Nowack JT, McCravy KW, Fetting CJ, Berisford CW (2001) Susceptibility of adult hymenopteran parasitoids of the Nantucket pine tip moth (Lepidoptera: Tortricidae) to broad-spectrum and biorational insecticides in a laboratory study. J Econ Entomol 94:1122–1129
Oatman ER, Platner GR (1971) Biological control of the tomato fruitworm, cabbage looper, and hornworms on processing tomatoes in southern California, using mass releases of Trichogramma pretiosum. J Econ Entomol 64:501–506
Orr DB, Boethel DJ, Layton MB (1989) Effect of insecticide applications in soybean on Trissolcus basalis (Hymenoptera: Scelionidae). J Econ Entomol 82:1078–1084
Saber M, Hassan SA, Hejazi MJ (2004) Effects of Azadirachin/Neemazal on different stages and adult life table parameters of Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). J Econ Entomol 97:905–910
SAS Institute (2010) The SAS system for Windows, release 10.1. Cary, N.C
Scholz BCG, Zalucki MP (2000) The effects of two new insecticides on the survival of adult Trichogramma pretiosum Riley in sweet corn. In: Austin A, Dowton M (eds) Hymenoptera: evolution, biodiversity and biological control. CSIRO, Collingwood, pp 381–387
Smith SM (1996) Biological control with Trichogramma: advances, successes, and potential of their use. Ann Rev Entomol 41:375–406
Sparks TC, Crouse GD, Durst G (2001) Natural products as insecticides: the biology, biochemistry and quantitative structure-activity relationships of spinosyns and spinosoids. Pest Manag Sci 57:896–905
Studebaker GE, Kring TJ (2003) Effects of insecticides on Orius insidiosus (Hemiptera: Anthocoridae), measured by field, greenhouse and petri dish bioassays. Fla Entomol 86:178–185
Suh CPC, Orr DB, Van Duyn JW (2000) Effect of insecticides on Trichogramma exiguum (Trichogrammatidae: Hymenoptera) preimaginal development and adult survival. J Econ Entomol 93:577–583
Thompson GD, Michel HH, Yao RC, Mynderse JS, Mosburg CT, Wordsen TV, Chio ET, Sparks TC, Hutchins SH (1997) The discovery of Saccharopolyspora spinosa and a new class of insect control products. Down Earth 52(1):1–5
Tomlin CDS (ed) (2006) The Pesticide Manual, A World Compendium, 14th ed. British Crop Protection Council, Hampshire
Varma GC, Singh PP (1987) Effect of insecticides on the emergence of Trichogramma brasiliensis (Hymenoptera: Trichogrammatidae) from parasitized host eggs. Entomophaga 32:443–448
Villanueva RT, Walgenbach JF (2005) Development, oviposition, and mortality of Neoseiulus fallacies (Acari: Phytoseiidae) in response to reduced-risk insecticides. J Econ Entomol 98:2114–2120
Williams T, Valle J, Viñuela E (2003) Is the naturally derived insecticide spinosad compatible with insect natural enemies? Biocontrol Sci Technol 13:459–475
Xu Y–Y, Liu T-X, Jones WA, Leibee G (2004) Effects of selected insecticides on Diadegma insulare, a parasitoid of diamondback moth on cabbage. Biocontrol Sci Technol 14:713–723
Acknowledgments
Our thanks to Jose M Martinez (Texas AgriLife Research, Texas A&M University System, Weslaco, Texas, USA) for his assistance. Our special thank extended to Dr Ed King (National Biological Control Laboratory, USDA-ARS, Stoneville, MS) for providing Trichogramma brassicae for this study, and to Dr John D Pinto (Department of Entomology, University of California-Riverside) for verification of the parasitoid species. We thank Texas AgriLife Research (TX-H8775) and Northwest A&F University (Yangling, Shaanxi, China) for financial support of this research (TXL-09-001-985-NWAFU). The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, TX., Zhang, Y. Side effects of two reduced-risk insecticides, indoxacarb and spinosad, on two species of Trichogramma (Hymenoptera: Trichogrammatidae) on cabbage. Ecotoxicology 21, 2254–2263 (2012). https://doi.org/10.1007/s10646-012-0981-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-012-0981-5