Abstract
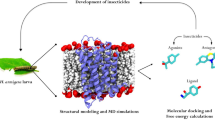
In insects, the process of molting and metamorphosis are mainly regulated by a steroidal hormone 20-hydroxyecdysone (20E) and its analogs (ecdysteroids) that specifically bind to the ecdysone receptor ligand-binding domain (EcR-LBD). Currently, several synthetic non-steroidal ecdysone agonists, including tebufenozide, are commercially available as insecticides. Tebufenozide exerts its activity by binding to the 20E-binding site and thus activating EcR permanently. It appears that subtle differences in the architecture among LBDs may underpin the differential binding affinity of tebufenozide across taxonomic orders. In brief, first we demonstrated the harmlessness of tebufenozide towards Chrysoperla externa (Ce). Then, a molecular analysis of EcR-LBD of two neuropteran insects Chrysoperla carnea and Ce was presented. Finally, we constructed a chrysopid in silico homology model docked ponasterone A (PonA) and tebufenozide into the binding pocket and analyzed the amino acids indentified as critical for binding to PonA and tebufenozide. Due to a restrict extent in the cavity at the bottom of the ecdysone-binding pocket a steric clash occurred upon docking of tebufenozide. The absence of harm biological effect and the docking results suggest that tebufenozide is prevented of any deleterious effects on chrysopids.





Similar content being viewed by others
References
Albuquerque GS, Tauber CA, Tauber MJ (1994) Chrysoperla externa (Neuroptera: Chrysopidae): life history and potential for biological control in Central and South America. Biol Control 4:8–13
Billas IML, Iwema T, Garnier JM, Mitschler A, Rochel N, Moras D (2003) Structural adaptability in the ligand-binding pocket of the ecdysone hormone receptor. Nature 426:91–96
Billas IML, Browning C, Lawrence MC, Graham LD, Moras D, Hill R (2009) The structure and function of ecdysone receptor. In: Smagghe G (ed) Ecdysone: structures and functions. Springer, Berlin, pp 335–360
Bonneton F, Zelus D, Iwema T, Robinson-Rechavi M, Laudet V (2003) Rapid divergence of the ecdysone receptor in Diptera and Lepidoptera suggests coevolution between EcR and USP-RXR. Mol Biol Evol 20:541–553
Bonneton F, Brunet FG, Kathirithamby J, Laudet V (2006) The rapid divergence of the ecdysone receptor is a synapomorphy for Mecopterida that clarifies the Strepsiptera problem. Insect Mol Biol 15:351–362
Bonneton F, Chaumot A, Laudet V (2008) Annotation of Tribolium castaneum receptors reveals an increase in evolutionary rate of a network controlling the ecdysone cascade. Insect Biochem Mol Biol 38:416–429
Carmichael JA, Lawrence MC, Graham LD, Pilling PA, Epa VC, Noyce L, Lovrecz G, Winkler DA, Pawlak-Skrzecz A, Eaton RE, Hannan GN, Hill RJ (2005) The X-ray structure of a hemipteran ecdysone receptor ligand-binding domain. J Biol Chem 280:22258–22269
Christiaens O, Iga M, Velardé RA, Rougé P, Smagghe G (2010) Halloween genes and nuclear receptors in ecdysteroid biosynthesis and signalling in the pea aphid. Insect Mol Biol 19:197–200
Dhadialla TS, Carlson GR, Le DP (1998) New insecticides with ecdysteroidal and juvenile hormone activity. Annu Rev Entomol 43:545–569
Dhadialla TS, Retnakaran A, Smagghe G (2005) Insect growth- and development-disrupting insecticides. In: Gilbert LI, Iatrou K, Gill SS (eds) Comprehensive molecular insect science, vol 6. Elsevier Press, Oxford, pp 55–116
Fahrbach SE, Smagghe G, Velardé RA (2012) Insect nuclear receptors. Annu Rev Entomol 57: doi:10.1146/annurev-ento-120710-100607
Ferreira AJ, Carvalho GA, Botton M, Mendonça LA, Corrêa ARB (2005) Selectivity of insecticides uses in apple orchards to eggs of Chrysoperla externa (Hagen) (Neuroptera: Chrysopidae). Cienc Rural 35:756–762
Giolo FP, Medina P, Grutzmacher AD, Viñuela E (2009) Effects of pesticides commonly used in peach orchards in Brazil on predatory lacewing Chrysoperla carnea under laboratory conditions. Biocontrol 54:625–635
Godoy MS, Carvalho GA, Moraes JC, Cosme LV, Gousain MM, Carvalho CF, Morais AA (2004a) Selectivity of six insectides used in citrus crops on pupae and adults of Chrysoperla externa (Hagen) (Neuroptera: Chrysopidae). Biol Control 33:359–364
Godoy MS, Carvalho GA, Moraes JC, Junior MG, Morais AA, Cosme LV (2004b) Selectivity of insecticides used in citrus crops to eggs and larvae of Chrysoperla externa (Hagen) (Neuroptera: Chrysopidae). Neotrop Entomol 33:639–646
Graham LD, Johnson WM, Pawlak-Skrzecz A, Eaton RE, Bliese M, Howell L, Hannan GN, Hill RJ (2007) Ligand binding by recombinant domains from insect ecdysone receptors. Insect Biochem Mol Biol 37:611–626
Graham LD, Johnson WM, Tohidi-Esfahani D, Pawlak-Skrzecz A, Bliese M, Lovrecz GO, Lu L, Howell L, Hannan GN, Hill RJ (2009) Ecdysone receptors of pest insects–molecular cloning, characterisation, and a ligand binding domain-based fluorescence polarization screen. In: Smagghe G (ed) Ecdysone: structures and functions. Springer, Berlin, pp 444–474
Iwema T, Billas IML, Beck Y, Bonneton F, Nierengarten H, Chaumot A, Richards G, Laudet V, Moras D (2007) Structural and functional characterization of a novel type of ligand-independent RXR-USP receptor. EMBO J 26:3770–3782
Johnson PD, Besselsen DG (2002) Practical aspects of experimental design in animal research. ILAR J 43:202–206
Landau M, Mayrose I, Rosenberg Y, Glaser F, Martz E, Pupko T, Ben-Tal N (2005) ConSurf 2005: the projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Res 33:w299–w302
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291
Mas MT, Smith KC, Yarmush DL, Aisaka K, Fine RM (1992) Modeling the anti-CEA antibody combining site by homology and conformational search. Proteins Struct Funct Genet 14:483–498
Medina P, Budia F, Tirry L, Smagghe G, Viñuela E (2001) Compatibility of spinosad, tebufenozide and azadirachtin with eggs and pupae of the predator Chrysoperla carnea (Stephens) under laboratory conditions. Bio Sci Technol 11:597–610
Medina P, Smagghe G, Budia F, del Estal P, Tirry L, Viñuela E (2002) Significance of penetration, excretion and transovarial uptake to toxicity of three insect growth regulators in predatory lacewing adults. Arch Insect Biochem Physiol 51:91–101
Medina P, Smagghe G, Budia F, Tirry L, Viñuela E (2003) Toxicity and absorption of azadirachtin, diflubenzuron, pyriproxyfen and tebufenozide after topical application in predatory larvae of Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae). Environ Entomol 32:196–203
Mommaerts V, Sterk G, Smagghe G (2006) Bumblebees can be used in combination with juvenile hormone analogues and ecdysone agonists. Ecotoxicology 15:513–521
Moras D, Gronemeyer H (1998) The nuclear receptor ligand-binding domain: structure and functions. Curr Opin Cell Biol 10:384–391
Nakagawa Y (2005) Nonsteroidal ecdysone agonists. Vitam Horm 73:131–173
Nakagawa Y, Henrich V (2009) Arthropods nuclear receptors and their role in molting. FEBS J 276:6128–6157
Ponder JW, Richards FM (1987) Tertiary templates for proteins: use of packing criteria in the enumeration of allowed sequences for different structural classes. J Mol Biol 193:775–791
Retnakaran A, Krell P, Feng Q, Arif B (2003) Ecdysone agonists: mechanism and importance in controlling insect pests of agriculture and forestry. Arch Insect Biochem Physiol 54:187–199
Rimoldi F, Schneider MI, Ronco AE (2008) Susceptibility of Chrysoperla externa eggs (Neuroptera: Chrisopidae) to conventional and biorational insecticides. Environ Entomol 37:1252–1257
Risler JL, Delorme MO, Delacroix H, Henaut A (1998) Amino acid substitutions in structurally related proteins. A pattern recognition approach. Determination of a new an efficient scoring matrix. J Mol Biol 204:1019–1029
Senior LJ, McEwen PK (2001) The use of lacewings in biological control. In: McEwan PK, New TR, Whittington AE (eds) Lacewings in the crop environment. Cambridge University Press, Cambridge, pp 296–302
Smagghe G, Degheele D (1993) Metabolism, pharmacokinetics and toxicity of the first nonsteroidal ecdysteroid agonist RH-5849 on Spodoptera exempta (Walker), Spodoptera exigua (Hübner), and Leptinotarsa decemlineata (Say). Pestic Biochem Physiol 46:149–160
Smagghe G, Degheele D (1994) Action of the nonsteroidal ecdysteroid mimic, RH-5992 on insects of different orders. Pestic Sci 42:85–92
Soin T, Iga M, Swevers L, Rougé P, Janssen CR, Smagghe G (2009) Towards Coleoptera-specific high-throughput screening systems for compounds with ecdysone activity: development of EcR reporter assays using weevil (Anthonomus grandis)-derived cell lines and in silico analysis of ligand binding to A. grandis EcR ligand-binding pocket. Insect Biochem Mol Biol 39:523–534
Soin T, Swevers L, Kotzia G, Iatrou K, Janssen CR, Rougé P, Harada T, Nakagawa Y, Smagghe G (2010) Comparison of the activity of non-steroidal ecdysone agonists between dipteran and lepidopteran insects, using cell-based EcR reporter assays. Pest Manag Sci 66:1215–1229
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL-X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tool. Nucleic Acids Res 15:4876–4882
Vogt H (1994) Effects of pesticides on Chrysoperla carnea Steph. (Neuroptera: Chrysopidae) in the field and comparison with laboratory and semi-field results. IOBC/WPRS Bull 17:71–82
Wing KD, Slawecki RA, Carlson GR (1988) RH-5849, a nonsteroidal ecdysone agonist: effects on larval Lepidoptera. Science 241:470–472
Yao TP, Forman BM, Jiang Z, Cherbas L, Chen JD, McKeown M, Cherbas P, Evans RM (1993) Functional ecdysone receptor is the product of EcR and ultraspiracle genes. Nature 366:476–479
Yong L, Lambert MH, Xu HE (2003) Activation of nuclear receptors: a perspective from structural genomics. Structure 11:714–746
Acknowledgments
Appreciations are expressed to the Funding Agency from the Brazilian Ministry of Education (CAPES) and to the National Council of Scientific and Technological Development (CNPq) for supporting a PhD grant to M.J. Zotti. This project is also supported by the Special Research Fund of Ghent University and the Fund for Scientific Research-Flanders (FWO-Vlaanderen, Belgium) to G. Smagghe.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zotti, M.J., Christiaens, O., Rougé, P. et al. Sequencing and structural homology modeling of the ecdysone receptor in two chrysopids used in biological control of pest insects. Ecotoxicology 21, 906–918 (2012). https://doi.org/10.1007/s10646-012-0852-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-012-0852-0