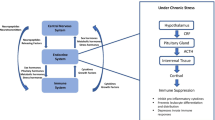
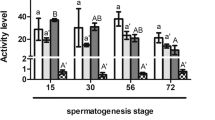
Abstract
During the last decade, a number of studies have shown that, in addition to their classically described reproductive function, estrogens and androgens also regulate the immune system in teleosts. Today, several molecules are known to interfere with the sex-steroid signaling. These chemicals are often referred to as endocrine disrupting contaminants (EDCs). We review the growing evidence that these compounds interfere with the fish immune system. These studies encompass a broad range of approaches from field studies to those at the molecular level. This integrative overview improves our understanding of the various endocrine-disrupting processes triggered by these chemicals. Furthermore, the research also explains why fish that have been exposed to EDCs are more sensitive to pathogens during gametogenesis. In this review, we first discuss the primary actions of sex-steroid-like endocrine disruptors in fish and the specificity of the fish immune system in comparison to mammals. Then, we review the known interactions between the immune system and EDCs and interpret the primary effects of sex steroids (estrogens and androgens) and their related endocrine disruptors on immune modulation. The recent literature suggests that immune parameters may be used as biomarkers of contamination by EDCs. However, caution should be used in the assessment of such immunotoxicity. In particular, more attention should be paid to the specificity of these biomarkers, the external/internal factors influencing the response, and the transduction pathways induced by these molecules in fish. The use of the well-known mammalian models provides a useful guide for future research in fish.

Similar content being viewed by others
References
Aluru N, Jorgensen EH, Maule AG, Vijayan MM (2004) PCB disruption of the hypothalamus-pituitary-interrenal axis involves brain glucocorticoid receptor downregulation in anadromous Arctic charr. Am J Physiol Regul Integr Comp Physiol 287:R787–R793
Ankley GT, Jensen KM, Makynen EA, Kahl MD, Korte JJ, Hornung MW, Henry TR, Denny JS, Leino RL, Wilson VS, Cardon MC, Hartig PC, Earl Gray L (2003) Effects of the androgenic growth promoter 17-β-trenbolone on fecundity and reproductive endocrinology of the fathead minnow. Environ Toxicol Chem 22:1350–1360
Ankley GT, Defoe DL, Kahl MD, Jensen KM, Makynen EA, Miracle A, Hartig P, Gray LE, Cardon M, Wilson V (2004) Evaluation of the model anti-androgen flutamide for assessing the mechanistic basis of responses to an androgen in the fathead minnow (Pimephales promelas). Environ Sci Technol 38:6322–6327
Ankley GT, Jensen KM, Durhan EJ, Makynen EA, Butterworth BC, Kahl MD, Villeneuve DL, Linnum A, Gray LE, Cardon M, Wilson VS (2005) Effects of two fungicides with multiple modes of action on reproductive endocrine function in the fathead minnow (Pimephales promelas). Toxicol Sci 86:300–308
Aoki T, Takano T, Santos MD, Kondo H, Hirono I (2008) Molecular innate immunity in teleost fish: review and future perspectives. In: Tsukamoto K, Kawamura T, Takeuchi T, Beard Jr. TD, Kaiser MJ (Eds) Fisheries for global welfare and environment. 5th World Fisheries Congress 263–276
Arukwe A (2001) Cellular and molecular responses to endocrine-modulators and the impact on fish reproduction. Mar Pollut Bull 42:643–655
Baldwin WS, Roling JA, Peterson S, Chapman LM (2005) Effects of nonylphenol on hepatic testosterone metabolism and the expression of acute phase proteins in winter flounder (Pleuronectes americanus): comparison to the effects of Saint John’s Wort. Comp Biochem Physiol C Toxicol Pharmacol 140:87–96
Baron D, Houlgatte R, Fostier A, Guiguen Y (2008) Expression profiling of candidate genes during ovary-to-testis trans-differenciation in rainbow trout masculinized by androgens. Gen Comp Endocrinol 156:369–378
Benninghoff AD, Williams DE (2008) Identification of a transcriptional fingerprint of estrogen exposure in rainbow trout liver. Toxicol Sci 101:65–80
Binuramesh C, Prabakaran M, Steinhagen D, Dinakaran M (2006) Effect of sex ratio on the immune system of Oreochromis mossambicus (Peters). Brain behav immun 20:300–308
Bols NC, Brubacher JL, Ganassin RC, Lee LEJ (2001) Ecotoxicology and innate immunity in fish. Dev Comp Immunol 25:853–873
Bowden TJ, Thompson KD, Morgan AL, Gratacap RML, Nikoskelainen S (2007) Seasonal variation and the immune response: a fish perspective. Fish Shellfish Immunol 22:695–706
Browser PR, Munson AD (1986) Seasonal variation in channel catfish virus antibody titers in adult channel catfish. Progress Fish Cult 48:198–199
Chaves-Pozo E, Liarte S, Fernández-Alacid L, Abellán E, Meseguer J, Mulero V, García-Ayala A (2008) Pattern of expression of immune-relevant genes in the gonad of a teleost, the gilthead seabream (Sparus aurata L.). Mol Immunol 45:2998–3011
Cheshenko K, Pakdel F, Segner H, Kah O, Eggen RI (2008) Interference of endocrine disrupting chemicals with aromatase CYP19 expression or activity, and consequences for reproduction of teleost fish. Gen Comp Endocrinol 155:31–62
Christiansen LB, Pedersen KL, Korsgaard B, Bjerregaard P (1998) Estrogenicity of xenobiotics in rainbow trout (Oncorhynchus mykiss) using in vivo synthesis of vitellogenin as a biomarker. Marine Environ Res 46:137–140
Cook J (1994) The effects of stress, background colour and steroid hormones on the lymphocytes of rainbow trout (Oncorhynchus mykiss). Ph.D. Thesis, University of Sheffield
Cosnefroy A, Brion F, Guillet B, Laville N, Porcher JM, Balaguer P, Aït-Aïssa S (2009) A stable fish reporter cell line to study estrogen receptor transactivation by environmental (xeno)estrogens. Toxicol In Vitro 23:1450–1454
Craig PM, Hogstrand C, Wood CM, McClelland GB (2009) Gene expression endpoints following chronic waterborne copper exposure in a genomic model organism, the zebrafish, Danio rerio. Physiol Genomics 40:23–33
Cuesta A, Vargas-Chacoff L, García-López A, Arjona FJ, Martínez-Rodríguez G, Meseguer J, Mancera JM, Esteban MA (2007) Effect of sex-steroid hormones, testosterone and estradiol, on humoral immune parameters of gilthead seabream. Fish Shellfish Immunol 23:693–700
Cuesta A, Meseguer J, Esteban MA (2008) Effects of the organochlorines p, p′-DDE and lindane on gilthead seabream leucocyte immune parameters and gene expression. Fish Shellfish Immunol 25:682–688
Cutolo M, Sulli A, Capellino S, Villagio B, Montagna P, Seriolo B, Straub RH (2004) Sex hormones influence on the immune system: basic and clinical aspects in autoimmunity. Lupus 13:635–638
Cutolo M, Capellino S, Montagna P, Ghiorzo P, Sulli A, Villagio B (2005) Sex hormone modulation of cell growth and apoptosis of the human monocytic/macrophage cell line. Arthritis Res Ther 7:1124–1132
Cutolo M, Capellino S, Sulli A, Serioli A, Secchi ME, Villaggio B, Straub RH (2006) Estrogens and autoimmune diseases. Ann NY Acad Sci 1089:538–545
Cutolo M, Montagna P, Brizzolara R, Sulli A, Seriolo B, Villagio B, Triolo P, Clerico P, Soldano S (2009) Sex hormones modulate the effects of leflunomide on cytokine production by cultures of differentiated monocyte/macrophages and synovial macrophages from rheumatoid arthritis patients. J Autoimmunity 32:254–260
Deane EE, Li J, Woo NY (2001) Hormonal status and phagocytic activity in sea bream infected with vibriosis. Comp Biochem Physiol B Mol Biol 129:687–693
Dorts J, Catherine A, Wright-Osment MK, Richter CA, Ellersieck MR, Carter BJ, Tillitt DE (2009) The genomic transcriptional response of female fathead minnows (Pimephales promelas) to an acute exposure to the androgen, 17beta-trenbolone. Aquat Toxicol 91:44–53
Duffy E, Carlson EA, Li Y, Prophete C, Zelikoff JT (2003) Age-related differences in the sensitivity of the fish immune response to a coplanar PCB. Ecotoxicology 12:251–259
Dunier M, Siwicki K (1993) Effects of pesticides and other organic pollutants in the aquatic environment on immunity of fish: a review. Fish Shellfish Immunol 8:423–438
Erlandsson MC, Ohlsson C, Gustafsson JA, Carlsten H (2001) Role of estrogen receptors alpha and beta in immune organ development and in estrogen-mediated effects on thymus. Immunology 103:17–25
Esteban A, Rodriguez A, Ayala AG, Meseguer J (2004) Effects of high doses of cortisol on innate cellular immune response of seabream (Sparus aurata L.). Gen Comp Endocrinol 137:89–98
Falk HF, Negele RD, Goerlich R (1990) Phagocytosis activity as an in vitro test for the effects of chronic exposure of rainbow trout to Linuron, a herbicide. J Appl Ichthyol 6:231–236
Farkas I, Varju P, Szabo E, Hrabovszky E, Okada N, Okada H, Liposits Z (2008) Estrogen enhances expression of the complement C5a receptor and the C5a-agonist evoked calcium influx in hormone secreting neurons of the hypothalamus. Neurochem Int 52:846–856
Fenske M, van Aerle R, Brack S, Tyler CR, Segner H (2001) Development and validation of a homologous zebrafish (Danio rerio) vitellogenin enzyme-linked immunosorbent assay (ELISA) and its application for studies on estrogenic chemicals. Comp Biochem Physiol C Toxicol Pharmacol 129:217–232
Fent K, Escher C, Caminada D (2006) Estrogenic activity of pharmaceuticals and pharmaceutical mixtures in a yeast reporter gene system. Reprod Toxicol 22:175–185
Filby AL, Thorpe KL, Maack G, Tyler CR (2007) Gene expression profiles revealing the mechanisms of anti-androgen- and estrogen-induced feminization in fish. Aquat Toxicol 81:219–231
Flores-Valverde AM, Horwood J, Hill EM (2010) Disruption of the steroid metabolome in fish caused by exposure to the environmental estrogen 17alpha-ethinylestradiol. Environ Sci Technol 44:3552–3558
Folmar LC, Hemmer MJ, Denslow ND, Kroll K, Chen J, Cheek A, Richman H, Meredith H, Grau EG (2002) A comparison of the estrogenic potencies of estradiol, ethynylestradiol, diethylstilbestrol, nonylphenol and methoxychlor in vivo and in vitro. Aquat Toxicol 60:101–110
Folomeev M, Dougados M, Beaune J, Kouyoumdjian JC, Nahoul K, Amor B, Alekberova Z (1992) Plasma sex hormones and aromatase activity in tissues of patients with systemic lupus erythematosus. Lupus 1:191–195
Förlin L, Anderson T (1984) Influence of biological and environmental factors on hepatic steroid and xenobiotic metabolism in fish: interaction with PCB and ß-naphtoflavone. Marine Environ Res 14:47–58
Garcia-Reyero N, Villeneuve DL, Kroll KJ, Liu L, Orlando EF, Watanabe KH, Sepúlveda MS, Ankley GT, Denslow ND (2009) Expression signatures for a model androgen and antiandrogen in the fathead minnow (Pimephales promelas) ovary. Environ Sci Technol 43:2614–2619
Grinwis GCM, Wester PW, Vethaak AD (2009) Histopathological effects of chronic aqueous exposure to bis(tri-n-butyltin)oxide (TBTO) to environmentally relevant concentrations reveal thymus atrophy in European flounder (Platichthys flesus). Environ Pollut 157:2587–2593
Gushiken Y, Watanuki H, Sakai M (2002) In vitro effect of carp phagocytic cells by bisphenol A and nonylphenol. Fish Sci 68:178–183
Harford AJ, O’Halloran K, Wright PFA (2005) The effects of in vitro pesticide exposures on the phagocytic function of four native Australian freshwater fish. Aquat Toxicol 75:330–342
Harford AJ, O’Halloran K, Wright PE (2007) Effect of in vitro and in vivo organotin exposures on the immune functions of murray cod (Maccullochella peelii peelii). Environ Toxicol Chem 26:1649–1656
Heppell SA, Denslow ND, Folmar LC, Sullivan CV (1995) Universal assay of vitellogenin as a biomarker for environmental estrogens. Environ Health Perspect 103:9–15
Hill EM, Evans KL, Horwood J, Rostkowski P, Olapado FO, Gibson R, Shears JA, Tyler JC (2010) Profiles and some initial identification of (anti)androgenic compounds in fish exposed to wastewater treatment works effluents. Environ Sci Technol 44:1137–1143
Hossain MS, Larsson A, Scherbak N, Olsson PE, Orban L (2008) Zebrafish androgen receptor: isolation, molecular, and biochemical characterization. Biol Reprod 78:361–369
Hou Y, Han XD (2001) Effects of estradiol-17beta on immunocompetence in rainbow trout. Acta Zool Sin 47:285–291
Hou Y, Suzuki Y, Aida K (1999a) Effects of steroids on the antibody producing activity of lymphocytes in rainbow trout. Fish Sci 65:850–855
Hou Y, Suzuki Y, Aida K (1999b) Changes in immunoglobulin producing cells in response to gonadal maturation in rainbow trout. Fish Sci 65:844–849
Hou Y, Suzuki Y, Aida K (1999c) Effects of steroid hormones on immunoglobulin M (IgM) in rainbow trout, Oncorhynchus mykiss. Fish Physiol Biochem 20:155–162
Islander U, Erlandsson MC, Hasséus B, Jonsson CA, Ohlsson C, Gustafsson JA, Dahlgren U, Carlsten H (2003) Influence of oestrogen receptor alpha and beta on the immune system in aged female mice. Immunology 110:149–157
Iwanowicz LR, Blazer VS, McCormick SD, Vanveld PA, Ottinger CA (2009) Aroclor 1248 exposure leads to immunomodulation, decreased disease resistance and endocrine disruption in the brown bullhead, Ameiurus nebulosus. Aquat Toxicol 93:70–82
Janele D, Lang T, Capellino S (2006) Effects of testosterone, 17-beta estradiol, and downstream estrogens on cytokine secretion from human leukocytes in the presence and absence of cortisol. Ann NY Acad Sci 1069:168–182
Jin Y, Chen R, Sun L, Liu W, Fu Z (2009) Photoperiod and temperature influence endocrine disruptive chemical-mediated effects in male adult zebrafish. Aquat Toxicol 92:38–43
Jobling S, Nolan M, Tyler CR, Brighty G, Sumpter JP (1998) Widespread sexual disruption in wild fish. Environ Sci Technol 32:2498–2506
Katsiadaki I, Scott AP, Hurst MR, Matthiessen P, Mayer I (2002) Detection of environmental Androgens: a novel method based on Enzyme-Linked Immunosorbent Assay of spiggin, the Stickleback (Gasterosteus Aculeatus) glue protein. Environ Toxicol Chem 21:1946–1954
Katsiadaki I, Morris S, Squires C, Hurst MR, James JD, Scott AP (2006) Use of the Three-Spined Stickleback (Gasterosteus aculeatus) as a sensitive in vivo test for detection of Environmental Antiandrogens. Environ Health Perspect 114:115–121
Kausch U, Alberti M, Haind S, Budczies J, Hock B (2008) Biomarkers for exposure to estrogenic compounds: gene expression analysis in Zebrafish (Danio rerio). Environ Toxicol 23:15–24
Kawano H, Kono T, Watanuki H, Savan R, Sakai M (2003) Analysis of genes expressed in head kidney of common carp Cyprinus carpio L. treated with cortisol. Comp Biochem Physiol B 136:875–886
Kloas W, Urbatzka R, Opitz R, Würtz S, Behrends T, Hermelink B, Hofmann F, Jagnytsch O, Kroupova H, Lorenz C, Neumann N, Pietsch C, Trubiroha A, Van Ballegooy C, Wiedemann C, Lutz I (2009) Endocrine disruption in aquatic vertebrates. Ann N Y Acad Sci 1163:187–200
Knudsen FR, Pottinger TG (1999) Interaction of endocrine disrupting chemical, singly and combination, with estrogen-, androgen-, and corticosteroid-binding sites in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 44:159–170
Korter R, Vainikka A (2008) Seasonality of innate immunity; evolutionary aspects and latest updates. In: Durand M, Morel CV (eds) New research on innate immunity. Nova Science Publishers, New York, pp 13–45
Kortner TM, Rocha E, Arukwe A (2009) Previtellogenic oocyte growth and transcriptional changes of steroidogenic enzyme genes in immature female Atlantic cod (Gadus morhua L.) after exposure to the androgens 11-ketotestosterone and testosterone. Comp Biochem Physiol A Mol Integr Physiol 152:304–313
Krøvel AV, Søfteland L, Torstensen BE, Olsvik PA (2010) Endosulfan in vitro toxicity in Atlantic salmon hepatocytes obtained from fish fed either fish oil or vegetable oil. Comp Biochem Physiol C Toxicol Pharmacol 151:175–186
Kumari J, Sahoo PK, Swain T, Sahoo SK, Sahu AK, Mohanty BR (2006) Seasonal variation in the innate immune parameters of the Asian catfish Clarias batrachus. Aquaculture 252:121–127
Kumari J, Larsen AN, Bogwald J, Dalmo RA (2009) Interleukin-17D in Atlantic salmon (Salmo salar): molecular characterization, 3D modelling and promoter analysis. Fish Shellfish Immunol 27:647–659
Kurtz J, Kalbe M, Langefors A, Mayer I, Milinski M, Hasselquist D (2007) An experimental test of the immunocompetence handicap hypothesis in a teleost fish: 11-ketotestosterone suppresses innate immunity in three-spined sticklebacks. Am Nat 170:509–519
Lamková K, Simková A, Palíková M, Jurajda P, Lojek A (2007) Seasonal changes of immunocompetence and parasitism in chub (Leuciscus cephalus), a freshwater cyprinid fish. Parasitol Res 101:775–789
Langston WJ, Burt GR, Chesman BS, Vane CH (2005) Partitioning, bioavailability and effects of oestrogens and xeno-estrogens in the aquatic environment. J Mar Biol Assoc UK 85:1–31
Larsson DGJ, Adolfsson-Erici M, Thomas P (2006) Characterization of putative ligands for a fish gonadal androgen receptor in a pulp mill effluent. Environ Toxicol Chem 25:419–427
Lavado R, Thibat R, Raldua D, Martin R, Porte C (2004) First evidence of endocrine disruption in feral carp from the Ebro river. Toxicol Appl Pharmacol 196:247–257
Law WY, Chen WH, Song YL, Dufour S, Chang CF (2001) Differential in vitro suppressive effects of steroids on leukocyte phagocytosis in two teleosts, tilapia and common carp. Gen Comp Endocrinol 121:163–172
Le Gac F, Thomas J-L, Mourot B, Loir M (2001) In vivo and in vitro effects of prochloraz and nonylphenol ethoxylates on trout spermatogenesis. Aquat toxicol 53:187–200
Legler J, Zeinstra LM, Schuitemaker F, Lanser PH, Bogerd J, Brouwer A, Vethaak AD, De Voogt P, Murk AJ, Van der Burg B (2002) Comparison of in vivo and in vitro reporter gene assays for short-term screening of estrogenicactivity. Environ Sci and Technol 36:4410–4415
Lehmann D, Siebold K, Emmons LR, Muller HJ (1988) Androgens inhibit proliferation of human peripheral blood lymphocytes in vitro. Clin Immunol Immunopath 46:122–128
Liu X, Su H, Zhu P, Zhang Y, Huang J, Lin H (2009) Molecular cloning, characterization and expression pattern of androgen receptor in Spinibarbus denticulatus. Gen Comp Endocrinol 160:93–101
Lorenzen K (1993) Acquired immunity o infectious diseases in fish: implications for the interpretation of fish disease surveys. In: Braunbeck T, Hanke W, Segner H (eds) Fish: ecotoxicology and ecophysiology. Verlag Chemie, Weinheim/New york, pp 183–196
Lye CM, Frid CLJ, Gill ME, Mc Cormick D (1997) Abnormalities in the reproductive health of flounder Platichthys flesus exposed to effluent from a sewage treatment works. Mar Pollut Bull 34:34–41
Lynn SG, Birge WJ, Shepherd BS (2008) Molecular characterization and sex-specific tissue expression of estrogen receptor alpha (esr1), estrogen receptor betaa (esr2a) and ovarian aromatase (cyp19a1a) in yellow perch (Perca flavescens). Comp Biochem Physiol B Biochem Mol Biol 149:126–147
Marchand-Geneste N, Cazaunau M, Carpy AJ, Laguerre M, Porcher JM, Devillers J (2006) Homology model of the rainbow trout estrogen receptor (rtERalpha) and docking of endocrine disrupting chemicals (EDCs). SAR QSAR Environ Res 17:93–105
Matthews J, Celius T, Halgren R, Zacharewski T (2000) Differential estrogen receptor binding of estrogenic substances: a species comparison. J Steroid Biochem Mol Biol 74:223–234
Maule AG, Jorgensen EH, Vijayan MM, Killie JEA (2005) Aroclor 1254 exposure reduces disease resistance and innate immune responses in fasted arctic charr. Environ Toxicol Chem 24:117–124
Milla S, Wang N, Mandiki R, Kestemont P (2009) Corticosteroids: friends or foes of teleost fish reproduction? Comp Biochem Physiol A Mol Integr Physiol. 153:242–251
Miller LL, Wang F, Palace VP, Hontela A (2007) Effects of acute and subchronic exposures to waterborne selenite on the physiological stress response and oxidative stress indicators in juvenile rainbow trout. Aquat Toxicol 83:263–271
Misiti S, Stigliano A, Borro M, Gentile G, Michienzi S, Cerquetti L, Bucci B, Argese N, Brunetti E, Simmaco M, Toscano V (2010) Proteomic profiles in hyperandrogenic syndromes. J Endocrinol Invest 33:156–164
Misumi I, Yada T, Leong JA, Schreck CB (2009) The effect of in vitro exposure to tributyltin on the immune competence of chinook Salmon (Oncorhynchus tshawytscha) leukocytes. Arch Environ contamin toxicol 56:229–237
Moens LN, Van der ven K, Remortel PV, Del-Favero J, De Coen WM (2006) Expression profiling of endocrine-disrupting coumpounds using a customized Cyprinus carpio cDNA microarray. Toxicol Sci 93:298–310
Moens LN, Soetaert A, van der Ven K, Del-Favero J, De Coen WM (2007) Use of suppression subtractive hybridization PCR for the development of cDNA arrays for the detection of endocrine disruption in carp (Cyprinus carpio). Comp Biochem Physiol D Genomics Proteomics 2:18–33
Morgan AL, Thompson KD, Auchinachie NA, Migaud H (2008) The effect of seasonality on normal haematological and innate immune parameters of rainbow trout Oncorhynchus mykiss L. Fish Shellfish Immunol 25:791–799
Mortensen AS, Arukwe A (2008) Estrogenic effect of dioxin-like aryl hydrocarbon receptor (AhR) agonist (PCB congener 126) in salmon hepatocytes. Mar Environ Res 66:119–120
Mortensen AS, Arukwe A (2009) Effects of tributyltin (TBT) on in vitro hormonal and biotransformation responses in Atlantic salmon (Salmo salar). J Toxicol Environ Health A 72:209–218
Munkittrick KR, McMaster ME, Portt CB, Van Der Kraak GJ, Smith IR, Dixon DG (1992) Changes in maturity, plasma sex steroid levels, hepatic mixed-function oxygenase activity, and the presence of external lesions in lake whitefish (Coregonus culpeaformis) exposed to bleached kraft mill effluent. Can J Fish Aquat Sci 49:1560–1569
Munkittrick KR, Servos MR, Van Der Kraak GJ, McMaster ME, Portt CB, Van Den Heuvel MR (1994) Survey of receiving-water environmental impacts associated with discharges from pulp mills. 2. Gonad size, liver size, hepatic EROD activity and plasma sex steroid levels in white sucker. Environ Toxicol Chem 13:1089–1101
Nakayama A, Kurokawa Y, Kawahara E, kitayoshi N, Harino H, Miyadai T, Seikai T, Kawai S (2005) The immunotoxic effects of tributyltin on non-specific biodefense system in rainbow trout (Oncorhynchus mykiss). Jpn J Environ Toxicol 8:23–35
Nakayama A, Kurokawa Y, Harino H, Kawahara E, Miyadai T, Seikai T, Kawai S (2007) Effects of tributyltin on the immune system of Japanese flounder (Paralichthys olivaceus). Aquat Toxicol 83:126–133
Noaksson E, Linderoth M, Bosveld ATC, Norrgren L, Zebühr Y, Balk L (2003a) Endocrine disruption in brook trout (Salvelinus fontinalis) exposed to leachate from a public refuse dump. Sci Total Environ 305:87–103
Noaksson E, Linderoth M, Bosveld ATC, Balk L (2003b) Altered steroid metabolism in several teleost species exposed to endocrine disrupting substances in refuse dump leachate. Gen Comp Endocrinol 134:273–284
Orlando EF, Davis WP, Guillette LJ Jr (2002) Aromatase activity in the ovary and brain of the eastern mosquitofish (Gambusia holbrooki) exposed to paper mill effluent. Environ Health Perspect 110:429–433
Parks LG, Lambright CS, Orlando EF, Guillette LJ Jr, Ankley GT, Gray LE Jr (2001) Masculinization of female mosquitofish in kraft mill effluent-contaminated fenholloway river water is associated with androgen receptor agonist activity. Toxicol Sci 62:257–267
Peters RE, Courtenay SC, Cagampan S, Hewitt ML, MacLatchy DL (2007) Effects on reproductive potential and endocrine status in the mummichog (Fundulus heteroclitus) after exposure to 17alpha-ethynylestradiol in a short-term reproductive bioassay. Aquat Toxicol 85:154–166
Petit F, Le Goff P, Cravedi JP, Valotaire Y, Pakdel F (1997) Two complementary bioassays for screening the estrogenic potency of xenobiotics: recombinant yeast for trout estrogen receptor and trout hepatocyte cultures. J Mol Endocrinol 19:321–335
Pietsch C, Neumann N, Knopf K, Wuertz S, Kloas W (2009) Progestogens cause immunosuppression of stimulated carp (Cyprinus carpio L.) leukocytes in vitro. Comp Biochem Physiol C Toxicol Pharmacol 150:16–24
Plouffe DA, Hanington PC, Walsh JG, Wilson EC, Belosevic M (2005) Comparison of select innate immune mechanisms of fish and mammals. Xenotransplantation 12:266–277
Powell DB, Palm RC, Skillman A, Godtfredsen K (2003) Immunocompetence of juvenile Chinook salmon against Listonella anguillarum following dietary exposure to Aroclor® 1254. Environ Toxicol Chem 22:285–295
Purdom CE, Hardiman PA, Bye VJ, Eno NC, Tyler CR, Sumpter JP (1994) Estrogenic effects of effluents from sewage treatment works. Chem Ecol 8:275–285
Rabitto IS, Alves Costa JRM, Silva de Assis HC, Pelletier E, Akaishi FM, Anjos A, Randi MAF, Oliveira Ribeiro CA (2005) Effects of dietary Pb(II) and tributyltin on neotropical fish, Hoplias malabaricus: histopathological and biochemical findings. Ecotox Environ Safe 60:147–156
Regala RP, Rice CD, Schwedler TE, Dorociak IR (2001) The effects of tributyltin (TBT) and 3,3′,4,4′,5-pentachlorobiphenyl (PCB-126) mixtures on antibody responses and phagocyte oxidative burst activity in channel catfish, Ictalurus punctatus. Arch Environ Contam Toxicol 40:386–391
Rhen T, Cidlowski CA (2006) Estrogens and glucocorticoids have opposing effects on the amount and latent activity of complement proteins in the rat uterus. Biol Reprod 74:265–274
Rice CD, Schlenk D (1995) Immune function and cytochrome P4501A activity after acute exposure to 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) in channel catfish. J Aquat Animal Health 7:195–204
Rice CD, Xiang Y (2000) Immune function, hepatic CYP1A, and reproductive biomarker responses in the gulf killifish, Fundulus grandis, during dietary exposures to endocrine disrupters. Mar Environ Res 50:163–168
Rice CD, Kergosin DH, Adams SM (1996) Innate immune functions as a bioindicator of pollution stress in fish. Ecotoxicol Environ Saf 33:186–192
Robertson LR, Iwanowicz LR, Marranca JM (2009) Identification of centrarchid hepcidins and evidence that 17β-estradiol disrupts constitutive expression of hepcidin-1 and inducible expression of hepcidin-2 in largemouth bass (Micropterus salmoides). Fish Shellfish Immunol 26:898–907
Ros AFH, Ferreira C, Serrão Santos R, Oliveira RF (2006) Regulation of immunocompetence by different androgen metabolites in a blenny with alternative reproductive tactics. J Exp Zool 305:986–994
Routledge EJ, White R, Malcolm G, Sumpter JP (2000) Differencial effects of xenoestrogens on coactivator recruitment by estrogens receptor (ER) a and ERb. J Biol Chem 275:35986–35993
Roy P, Salminen H, Koskimies P, Simola J, Smeds A, Saukko P, Huhtaniemi IT (2004) Screening of some anti-androgenic endocrine disruptors using a recombinant cell-based in vitro bioassay. J Steroid Biochem Mol Biol 88:157–166
Ruggeri B, Ubaldi M, Lourdusamy A, Soverchia L, Ciccocioppo R, Hardiman G, Bakere ME, Palermo F, Polzonetti-Magni AM (2008) Variation of the genetic expression pattern after exposure to estradiol-17β and 4-nonylphenol in male zebrafish (Danio rerio). Gen Comp Endocrinol 158:138–144
Saha NR, Usami T, Suzuki Y (2004) In vitro effects of steroid hormones on IgM-secreting cells and IgM secretion in common carp (Cyprinus carpio). Fish Shellfish Immunol 17:149–158
Sanchez W, Goin C, Brion F, Olsson PE, Goksøyr A, Porcher JM (2008) A new ELISA for the three-spined stickleback (Gasterosteus aculeatus L.) spiggin, using antibodies against synthetic peptide. Comp Biochem Physiol C Toxicol Pharmacol 147:129–137
Saurabh S, Sahoo PK (2008) Lysozyme: an important defence molecule of fish innate immune system. Aqua Res 39:223–239
Scholz S, Klüver N (2009) Effects of endocrine disrupters on sexual, gonadal development in fish. Sex Dev 3:136–151
Schwaiger J, Spieser OH, Bauer C, Ferling H, Mallow U, Kalbfus W, Negele RD (2000) Chronic toxicity of nonylphenol and ethinylestradiol: haematological and histopathological effects in juvenile Common carp (Cyprinus carpio). Aquatic Toxicol 51:69–78
Shimasaki Y, Kitano T, Oshima Y, Inoue S, Imada N, Honjo T (2003) Tributyltin causes masculinization in fish. Environ Toxicol Chem 22:141–144
Shved N, Berishvili G, Häusermann E, D’Cotta H, Baroiller JF, Eppler E (2009) Challenge with 17alpha-ethinylestradiol (EE2) during early development persistently impairs growth, differentiation, and local expression of IGF-I and IGF-II in immune organs of tilapia. Fish Shellfish Immunol 26:524–530
Sieroslawska A, Siwicki AK (2003) In vitro influence of ciprofloxacin on selected functions of rainbow trout (Oncorhynchus mykiss) leucocytes. Pol J Vet Sci 6:47–48
Šimková A, Lafond T, Ondračková M, Jurajda P, Ottová E, Morand S (2008) Parasitism, life history traits and immune defence in cyprinid fish from Central Europe. BMC Evol Biol 8:29
Slater CH, Schreck CB (1997) Physiological levels of testosterone kill salmonid leukocytes in vitro. Gen Comp Endocrinol 106:113–119
Slater CH, Fitzpatrick MS, Schreck CB (1995) Characterization of an androgen receptor in salmonid lymphocytes: possible link to androgen-induced immunosuppression. Gen Comp Endocrinol 100:218–225
Smolinsky AN, Doughman JM, Kratzke LT, Lassiter CS (2010) Zebrafish (Danio rerio) androgen receptor: sequence homology and up-regulation by the fungicide vinclozolin. Comp Biochem Physiol C Toxicol Pharmacol 151:161–166
Sohoni P, Sumpter JP (1998) Several environmental oestrogens are also anti-androgens. J Endocrinol 158:327–339
Sone K, Hinago M, Itamoto M, Katsu Y, Watanabe H, Urushitani H, Tooi O, Guillette LJ Jr, Iguchi T (2005) Effects of an androgenic growth promoter 17beta-trenbolone on masculinization of Mosquitofish (Gambusia affinis affinis). Gen Comp Endocrinol 143:151–160
Steine NO, Melingen GO, Wergeland HI (2001) Antibodies against Vibrio salmonicida lipopolysaccharide (LPS) and wholebacteria in sera from Atlantic salmon (Salmo salar L.) vaccinated during the smolting and early post-smolt period. Fish shellfish immunol 11:39–52
Sumpter JP (2008) The ecotoxicology of hormonally active micropollutants. Water Sci Technol 57:125–130
Sunyer JO, Tort L, Lambris JD (1997) Structural C3 diversity in fish: characterization of five forms of C3 in the diploid fish Sparus aurata. J Immunol 158:2813–2821
Suzuki Y, Orito M, Iigo M, Kezuka H, Kobayashi M, Aida K (1996) Seasonal changes in blood IgM levels in goldfish, with special reference to water temperature and gonadal maturation. Fish Sci 62:754–759
Suzuki Y, Otaka T, Sato S, Hou YY, Aida K (1997) Reproduction related immunoglobulin changes in rainbow trout. Fish Physiol Biochem 17:415–421
Swain P, Kayak SK (2009) Role of maternally derived immunity in fish. Fish Shellfish Immunol 24:89–99
Tavares-Dias (2006) Cytochemical method for staining fish basophils. J Fish Biol 69:312–317
Tellez-Bañuelos MC, Santerre A, Casas-Solis J, Bravo-Cuellar A, Zaitsev G (2009) Oxidative stress in macrophages from spleen of Nile tilapia (Oreochromis niloticus) exposed to sublethal concentration of endosulfan. Fish Shellfish Immunol 27:105–111
Tellez-Bañuelos MC, Santerre A, Casas-Solis J, Zaitsev G (2010) Endosulfan increases seric interleukin-2 like (IL-2L) factor and immunoglobulin M (IgM) of Nile tilapia (Oreochromis niloticus) challenged with Aeromona hydrophila. Fish Shellfish Immunol 28:401–405
Thibaut R, Porte C (2004) Effects of endocrine disrupters on sex steroid synthesis and metabolism pathways in fish. J Steroid Biochem Mol Biol 92:485–494
Thilagam H, Gopalakrishnan S, Bo J, Wang KJ (2009) Effect of 17beta-estradiol on the immunocompetence of Japanese sea bass (Lateolabrax japonicus). Environ Toxicol Chem 28:1722–1731
Thorpe KI, Hutchinson TH, Hetheridge MJ, Sumpter JP, Tyler CR (2000) Development of an in vivo screening assay for estrogenic chemicals using juvenile rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem 19:2812–2820
Todo T, Ikeuchi T, Kobayashi T, Nagahama Y (1999) Fish androgen receptor: cDNA cloning, steroid activation of transcription in transfected mammalian cells, and tissue mRNA levels. Biochem Biophys Res Com 254:378–383
Tokalov SV, Gutzeit HO (2007) Lectin-binding pattern as tool to identify and enrich specific primary testis cells of the tilapia (Oreochromis niloticus) and medaka (Oryzias latipes). J Exp Zool B Mol Dev Evol 308:127–138
Tollefsen KE (2007) Binding of alkylphenols and alkylated non-phenolics to the rainbow trout (Oncorhynchus mykiss) plasma sex steroid-binding protein. Ecotoxicol Environ Saf 68:40–48
Tollefsen KE, Meys JFA, Frydenlund J, Stenersen J (2002) Environmental estrogens interact with and modulate the properties of plasma sex steroid-binding proteins in juvenile Atlantic salmon (Salmo salar). Mar Environ Res 54:697–701
Tollefsen KE, Finne EF, Romstad R, Sandberg C (2006) Effluents from oil production activities contain chemicals that interfere with normal function of intra- and extra-cellular estrogen binding proteins. Mar Environ Res 62:S191–S194
Tyler CR, van der Eerden B, Jobling S, Panter G, Sumpter JP (1996) Measurement of vitellogenin, a biomarker for exposure to oestrogenic chemicals, in a wide variety of cyprinid fish. J Comp Physiol B 166:418–426
Vaccaro E, Meucci V, Intorre L, Soldani G, Di Bello D, Longo V, Gervasi PG, Pretti C (2005) Effects of 17beta-estradiol, 4-nonylphenol and PCB 126 on the estrogenic activity and phase 1 and 2 biotransformation enzymes in male sea bass (Dicentrarchus labrax). Aquat Toxicol 75:293–305
Vainikka A, Jokinen EI, Kortet R, Paukku S, Pirhonen J, Rantala MJ, Taskinen J (2005) Effects of testosterone and b-glucan on immune functions in tench. J Fish Biol 66:348–361
Van den Belt K, Berckmans P, Vangenechten C, Verheyen R, Witters H (2004) Comparative study on the in vitro/in vivo estrogenic potencies of 17beta-estradiol, estrone, 17alpha-ethynylestradiol and nonylphenol. Aquat Toxicol 66:183–195
Van Ginneken V, Palstra A, Leonards P, Nieveen M, van den Berg H, Flik G, Spanings T, Niemantsverdriet P, van den Thillart G, Murk A (2009) PCBs and the energy cost of migration in the European eel (Anguilla anguilla L.). Aquat Toxicol 92:213–220
Vetillard A, Bailhache T (2006) Effects of 4-n-nonylphenol and tamoxifen on sGnRH, estrogen receptor and vitellogenin gene expression in juvenile rainbow trout. Toxicol Scie 92:537–544
Wang R, Belosevic M (1994) Estradiol increases susceptibility of goldfish to Trypanosoma danilewskyi. Dev Comp Immunol 18:377–387
Wang KJ, Cai JJ, Cai L, Qu HD, Yang M, Zhang M (2009) Cloning and expression of a hepcidin gene from a marine fish (Pseudosciaena crocea) and the antimicrobial activity of its synthetic peptide. Peptides 30:638–646
Wang RL, Bencic D, Villeneuve DL, Ankley GT, Lazorchak J, Edwards S (2010) A transcriptomic biological framework for studying mechanisms of endocrine disruption in small fish species. Aquatic toxicol 98:230–234
Wartman CA, Hogan NS, Hewitt LM, McMaster ME, Landman MJ, Taylor S, Kovacs TG, van den Heuvel MR (2009) Androgenic effects of a Canadian bleached kraft pulp and paper effluent as assessed using threespine stickleback (Gasterosteus aculeatus). Aquat Toxicol 92:131–139
Watanuki H, Yamaguchi T, Sakai M (2002) Suppression in function of phagocytic cells in common carp Cyprinus carpio L. injected with estradiol, progesterone or 11-ketotestosterone. Comp Biochem Physiol C Toxicol Pharmacol 132:407–413
Watanuki H, Gushiken Y, Sakai M (2003) In vitro modulation of common carp (Cyprinus carpio L.) phagocytic cells by Di-n-butyl phthalate and Di-2-ethylhexyl phthalate. Aquat Toxicol 63:119–126
Werner J, Ouellet JD, Cheng CS, Ju YJ, Law RD (2010) Pulp and paper mill effluents induce distinct gene expression changes linked to androgenic and estrogenic responses in the fathead minnow (Pimephales Promelas). Environ Toxicol Chem 29:430–439
Williams TD, Amer MD, George SG, Sabine V, Chipman JK (2007) Gene expression responses of European flounder (Platichtys flesus) to 17-beta estradiol. Toxicol Lett 168:236–248
Yamaguchi T, Watanuki H, Sakai M (2001) Effects of estradiol, progesterone and testosterone on the function of carp, Cyprinus carpio, phagocytes in vitro. Comp Biochem Physiol C Toxicol Pharmacol 129:49–55
Yin DQ, Hu SQ, Gu Y, Wei L, Liu SS, Zhang AQ (2007) Immunotoxicity of bisphenol A to Carassius auratus lymphocytes and macrophages following in vitro exposure. J Environ Sci 19:232–237
Yu Y, Zhong Q, Li C, Jiang L, Yan F, Wang Z, Zhang Q (2009) Isolation and characterization of Toll-like receptor 9 in half-smooth tongue sole Cynoglossus semilaevis. Fish Shellfish Immunol 26:492–499
Yum S, Woo S, Kagami Y, Park HS, Ryu JC (2010) Changes in gene expression profile of medaka with acute toxicity of Arochlor 1260, a polychlorinated biphenyl mixture. Comp Biochem Physiol C Toxicol Pharmacol 151:51–56
Zhang Z, Hu J (2008) Effects of p,p′-DDE exposure on gonadal development and gene expression in Japanese medaka (Oryzias latipes). J Environ Scie 20:347–352
Zhang X, Hecker M, Park JW, Tompsett AR, Newsted J, Nakayama K, Jones PD, Au D, Kong R, Wu RS, Giesy JP (2008) Real-time PCR array to study effects of chemicals on the Hypothalamic-Pituitary-Gonadal axis of the Japanese medaka. Aquat Toxicol 88:173–182
Acknowledgments
S.M. was supported by the Belgian National Funds for Scientific Research (FNRS), contract 2. 4570. 06.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Milla, S., Depiereux, S. & Kestemont, P. The effects of estrogenic and androgenic endocrine disruptors on the immune system of fish: a review. Ecotoxicology 20, 305–319 (2011). https://doi.org/10.1007/s10646-010-0588-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-010-0588-7