Abstract
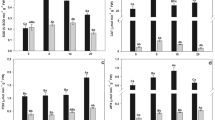
The accumulation of arsenic (As) and physiological responses of Lemna minor L. under different concentration (0, 1, 4, 16 and 64 μM) and duration (1, 2, 4 and 6 days) of two species As, NaAsO2 and Na2HAsO4·7H2O, were studied in hydroponics. The accumulation of both As species depended on As concentration and exposure duration. The highest accumulation of As was found as 17408 and 8674 μg g−1, for plants exposed to 64 μM of As(III) and As(V), respectively, after 6 days. Two-way ANOVA analyses indicated that, for plants exposed to arsenite (As(III)), exposure duration had a greater effect than concentration on As accumulation. Conversely, exposure concentration had a greater effect on As accumulation in plants exposed to arsenate (As(V)). Arsenic exposure levels, approaching 16 μM for As(III) and 64 μM for As(V), did not significantly affect EC values. Beyond these exposure concentrations, EC values increased in a manner that depended on duration. Significant effect of As(III) on lipid peroxidation was observed at 1 μM application whereas, this effect started to be significant after an exposure to 16 μM As(V). For both As(III) and As(V), photosynthetic pigment levels slightly increased for the first day with respect to the control, followed by a gradual decline at higher concentrations and durations. An increase in protein content and enzyme activity was observed at moderate exposure conditions, followed by a decrease. Significant positive correlations were determined between accumulated As and ion leakage and lipid peroxidation. Negative correlations were found between accumulated As and total chlorophyll and protein content. Our results suggested that exposure duration and concentration had a strong synergetic effect on antioxidant enzyme activity. The findings of the present study may be useful when this plant is used as a phytoremediator in arsenic-polluted water.


Similar content being viewed by others
References
Abbas MHH, Meharg AA (2008) Arsenate, arsenite and dimethyl arsinic acid (DMA) uptake and tolerance in maize (Zea mays L.). Plant Soil 304:277–289
Abedin MJ, Feldmann J, Meharg AA (2002) Uptake kinetics of arsenic species in rice (Oryza sativa L.) plants. Plant Physiol 128:1120–1128
Aebi H (1974) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie, Weinheim, pp 673–684
Alvarado S, Guédez M, Lué-Merú MP, Nelson G, Alvaro A, Jesús AC, Gyula Z (2008) Arsenic removal from waters by bioremediation with the aquatic plants Water Hyacinth (Eichhornia crassipes) and Lesser Duckweed (Lemna minor). Bioresour Technol 99:8436–8440
Arnon DI (1949) Copper enzyme in isolated chloroplast polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Cakmak I (2000) Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol 146:185–205
Calabrese EJ, Baldwin LA (2003) Hormesis: environmental and biomedical perspective. Crit Rev Toxicol 133:213–467
Cao X, Ma LQ, Tu C (2004) Antioxidative responses to arsenic in the arsenic-hyperaccumulator Chinese brake fern (Pteris vittata L.). Environ Pollut 128:317–325
Chakrabarty D, Trivedi PK, Misra P, Tiwari M, Shri M, Shukla D, Kumar S, Rai A, Pandey A, Nigam D, Tripathi RD, Tuli R (2009) Comparative transcriptome analysis of arsenate and arsenite stresses in rice seedlings. Chemosphere 74:688–702
Charlier HA, Albertson C, Thornock C, Warner L, Hurst T, Ellis R (2005) Comparison of the effects of arsenic (V), cadmium (II), and mercury (II) single metal and mixed metal exposure in radish, Raphanus sativus, Fescue grass, Festuca ovina, and duckweed, Lemna minor. Bull Environ Contam Toxicol 75:474–481
Choo TP, Lee CK, Low KS, Hishamuddin O (2006) Accumulation of chromium (VI) from aqueous solutions using water lilies (Nymphaea spontanea). Chemosphere 62:961–967
Dazy M, Béraud E, Cotelle S, Meux E, Masfaraud JF, Férard JF (2008) Antioxidant enzyme activities as affected by trivalent and hexavalent chromium species in Fontinalis antipyretica Hedw. Chemosphere 73:281–290
Devi SR, Prasad MNV (1998) Copper toxicity in Ceratophyllum demersum L. (Coontail), a free floating macrophyte: response of antioxidant enzymes and antioxidants. Plant Sci 13:157–165
Harris ED (1992) Regulation of antioxidant enzymes. FASEB J 6:2675–2683
Hartley-Whitaker J, Ainsworth G, Meharg AA (2001) Copper and arsenate-induced oxidative stress in Holcus lanatus L. clones with differential senstivity. Plant Cell Environ 24:713–722
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hou W, Chen X, Song G, Wang Q, Chang CC (2007) Effects of copper and cadmium on heavy metal polluted waterbody restoration by duckweed (Lemna minor). Plant Physiol Biochem 45:62–69
Khan I, Ahmad A, Iqbal M (2009) Modulation of antioxidant defence system for arsenic detoxification in Indian mustard. Ecotoxicol Environ Saf 72:626–634
Li WX, Chen TB, Huang ZC, Lei M, Liao XY (2006) Effect of arsenic on chloroplast ultrastructure and calcium distribution in arsenic hyperaccumulator Pteris vittata L. Chemosphere 62:803–809
Lichtenthaler H, Wellburn AR (1985) Determinationsof total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Lombi E, Zhao FJ, Fuhrmann M, Ma LQ, McGrath SP (2002) Arsenic distribution and speciation in the fronds of the hyperaccumulator Pteris vittata. New Phytol 156:195–203
Lowry OH, Roenbrough NJ, Farr AL, Randal EJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
MacFarlane GR, Burchett MD (2002) Toxicity, growth and accumulation relationships of copper, lead and zinc in the grey mangrove Avicennia marina (Forsk.) Vierh. Mar Environ Res 54:65–84
Meharg AA, Macnair MR (1990) An altered phosphate uptake system arsenate-tolerant Holcus lanatus L. New Phytol 116:29–35
Mkandawire M, Dudel EG (2005) Accumulation of arsenic in Lemna gibba L. (duckweed) in tailing waters of two abandoned uranium mining sites in Saxony, Germany. Sci Total Environ 336:81–89
Mkandawire M, Lyubun YV, Kosterin PV, Dudel EG (2004) Toxicity of arsenic species to Lemna gibba L. and the influence of phosphate on arsenic bioavailability. Environ Toxicol 19:26–34
Mrak T, Šlejkovec Z, Jeran Z, Jaćimović R, Kastelec D (2008) Uptake and biotransformation of arsenate in the lichen Hypogymnia physodes (L.) Nyl. Environ Pollut 151:300–307
Mylona PV, Polidoros AN, Scandalios JG (1998) Modulation of antioxidant responses by arsenic in maize. Free Radic Biol Med 25:576–585
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Naumann B, Eberius M, Appenroth KJ (2007) Growth rate based dose–response relationships and EC-values of ten heavy metals using the duckweed growth inhibition test (ISO 20079) with Lemna minor L. clone St. J Plant Physiol 164:1656–1664
Rahman AM, Hasegawa H, Ueda K, Maki T, Okumura C, Rahman MM (2007) Arsenic accumulation in duckweed (Spirodela polyrhiza L.): a good option for phytoremediation. Chemosphere 69:493–499
Rahman AM, Hasegawa H, Ueda K, Maki T, Rahman MM (2008) Influence of EDTA and chemical species on arsenic accumulation in Spirodela polyrhiza L. (duckweed). Ecotoxicol Environ Saf 70:311–318
Requejo R, Tena M (2005) Proteome analysis of maize roots reveals that oxidative stress is a main contributing factor to plant arsenic toxicity. Phytochemistry 66:1519–1528
Robinson B, Kim N, Marchetti M, Moni C, Schroeter L, Dijssel C, Milne G, Clothier B (2006) Arsenic hyperaccumulation by aquatic macrophytes in the Taupo Volcanic Zone, New Zealand. Environ Exp Bot 58:206–215
Seth CS, Chaturvedi PK, Misra V (2007) Toxic effect of arsenate and cadmium alone and in combination on giant duckweed (Spirodela polyrrhiza L.) in response to its accumulation. Environ Toxicol 22:539–549
Sharma P, Dubey RS (2005) Lead toxicity in plants. Braz J Plant Physiol 17:35–52
Singh N, Ma LQ, Srivastava M, Rathinasabapathi B (2006) Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L. and Pteris ensiformis L. Plant Sci 170:274–282
Sizova OI, Kochetkov VV, Validov SZ, Boronin AM, Kosterin PV, Lyubun YV (2002) Arsenic-contaminated soils: genetically modified Pseudomonas spp. and their arsenic-phytoremediation potential. J Soil Sediment 2:19–23
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Smith PG, Koch I, Reimer KJ (2008) Uptake, transport and transformation of arsenate in radishes (Raphanus sativus). Sci Total Environ 390:188–197
Srivastava S, Mishra S, Tripathi RD, Dwivedi S, Gupta DK (2006) Copper-induced oxidative stress and responses of antioxidants and phytochelatins in Hydrilla verticillata (L.f.) Royle. Aquat Toxicol 8:405–415
Srivastava S, Mishra S, Tripathi RD, Dwivedi S, Trivedi PK, Tandon PK (2007) Phytochelatins and antioxidant systems respond differentially during arsenite and arsenate stress in Hydrilla verticillata (L.f.) Royle. Environ Sci Technol 41:2930–2936
Tripathi RD, Srivastava S, Mishra S, Singh N, Tuli R, Gupta DK, Maathuis FJM (2007) Arsenic hazards: strategies for tolerance and remediation by plants. Trends Biotechnol 25:158–165
Visoottiviseth P, Francesconi K, Sridokchan W (2002) The potential of Thai indigenous plant species for the phytoremediation of arsenic contaminated land. Environ Pollut 118:453–461
Wang J, Zhao FJ, Meharg AA, Raab A, Feldmann J, McGrath SP (2002) Mechanisms of arsenic hyperaccumulation in Pteris vittata. Uptake kinetics, interactions with phosphate, and arsenic speciation. Plant Physiol 130:1552–1561
WHO (2001) Environmental health criteria 224, arsenic and arsenic compounds. Inter-organization programme for the sound management of chemicals, Geneva
Zhang W, Cai Y, Tu C, Ma LQ (2002) Arsenic speciation and distribution in an arsenic hyperaccumulating plant. Sci Total Environ 300:167–177
Zhang FQ, Wang YS, Lou ZP, Dong JD (2007) Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 67:44–50
Zhang X, Lin AJ, Zhao FJ, Xu GZ, Duan GL, Zhu YG (2008) Arsenic accumulation by the aquatic fern Azolla: comparison of arsenate uptake, speciation and efflux by A. caroliniana and A. filiculoides. Environ Pollut 156:1149–1155
Acknowledgements
The authors are deeply grateful for the technical assistance of Fuat Bozok and Musa Kar. This study was supported by Erciyes University Scientific Research Project Fund (FBA 07-32).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duman, F., Ozturk, F. & Aydin, Z. Biological responses of duckweed (Lemna minor L.) exposed to the inorganic arsenic species As(III) and As(V): effects of concentration and duration of exposure. Ecotoxicology 19, 983–993 (2010). https://doi.org/10.1007/s10646-010-0480-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-010-0480-5