Abstract
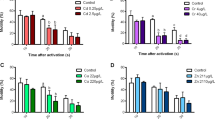
Juveniles female goldfish were exposed to atrazine (2-chloro-4-ethylamino-6-isopropylamino-s-triazine) at high doses, 100 and 1000 μg l−1 during 56 days in order to evaluate the potential action of the herbicide as an endocrine disruptor. Plasma concentration of estradiol (E2) and 11-ketotestosterone (11-KT) as well as activity and expression of aromatase in the gonads were evaluated. These parameters were completed with morphological measures such as gonadosomatic index (GSI) and histological analyses of gonads. Morphological parameters at both 100 and 1000 μg l−1 did not show any significant differences with the control groups. Correlated to the pathway hypothesized, no time-, dose-related effects were detected on the aromatase activity and the expression in the gonads of juvenile female goldfish. The same conclusion was attributed regarding the circulating E2 where no perceptible variation was detected. Nevertheless, a hormonal imbalance was detected for plasma concentration of the sex steroid 11-KT of fish exposed to 1000 μg l−1 after 56 days exposure. In these particular experimental conditions, we failed to demonstrate an effect of atrazine through the induction of aromatase and hormonal imbalance associated.





Similar content being viewed by others
References
Babic-Gojmerac T, Kniewald Z, Kniewald J (1989) Testosterone metabolism in neuroendocrine organs in male rats under atrazine and deethylatrazine influence. J Steroid Biochem 33:141–146
Coady KK, Murphy MB, Villeneuve DL, Hecker M, Jones PD, Carr JA, Solomon KR, Smith EE, Van Der Kraack G, Kendall RJ, Giesy JP (2005) Effects of atrazine on metamorphosis, growth, laryngeal and gonadal development, aromatase activity, and sex steroid concentrations in Xenopus laevis. Ecotoxicol Environ Saf 62:160–173
Connor K, Howell J, Chen I, Liu H, Berhane K, Sciarretta C, Safe S, Zacharewski T (1996) Failure of chloro-S-triazine-derived compounds to induce estrogen receptor-mediated responses in vivo and in vitro. Fundam Appl Toxicol 30:93–101
Crain DA, Guillette LJ Jr, Rooney AA, Pickford DB (1997) Alterations in steroidogenesis in alligators (Alligator mississippiensis) exposed naturally and experimentally to environmental contaminants. Environ Health Perspect 105:528–533
Crain DA, Spiteri ID, Guillette LJ Jr (1999) The functional and structural observations of the neonatal reproductive system of alligators exposed in ovo to atrazine, 2,4-D, or estradiol. Toxicol Ind Health 15:180–185
Eldridge JC, Wetzel LT, Tyrey L (1999) Estrous cycle patterns of Sprague-Dawley rats during acute and chronic atrazine administration. Reprod Toxicol 13:491–499
Fostier A, Jalabert B (1986) Steroidogenesis in rainbow trout (Salmo gairdneri) at various preovulatory stages: changes in plasma hormone levels and in vivo and in vitro responses of the ovary to salmon gonadotropin. Fish Physiol Biochem 2:87–99
Gonzales A, Piferrer F (2002) Characterization of aromatase activity in the sea bass: effects of temperature and different catalytic properties of brain and ovarian homogenates and microsomes. J Exp Zool 293:500–510
Gonzales A, Piferrer F (2003) Aromatase activity in the European sea bass (Dicentrarchus labrax L.) brain. Distribution and changes in relation to age, sex, and the annual reproductive cycle. Gen Comp Endocrinol 132:223–230
Gray LEJ, Kelce W, Wiese T (1997) Endocrine screening methods workshop report: detection of estrogenic & androgenic hormonal and antihormonal activity for chemicals that act via receptor or steroidogenic enzyme mechanisms. Reprod Toxicol 11:719–750
Hayes TB, Collins A, Lee M, Mendoza M, Noriega N, Stuart AA, Vonk A (2002) Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc Natl Acad Sci USA 99:5476–5480
Hecker M, Newsted JL, Murphy MB, Higley EB, Jones PD, Wu R, Giesy JP (2006) Human adrenocarcinoma (H295R) cells for rapid in vitro determination of effects on steroidogenesis: hormone production. Toxicol Appl Pharmacol 217:114–124
Heneweer M, Van den Berg M, Sanderson JT (2004) A comparison of human H295R and rat R2C cell lines as in vitro screening tools for effects on aromatase. Toxicol Lett 146:183–194
Hinfray N, Porcher JM, Brion F (2006) Inhibition of rainbow trout (Oncorhynchus mykiss) P450 aromatase activities in brain and ovarian microsomes by various environmental substances. Comp Biochem Physiol C Toxicol Pharmacol 144:252–262
Holloway AC, Stys KA, Foster WG (2005) DDE-induced changes in aromatase activity in endometrial stromal cells in culture. Endocrine 27:45–50
Huber W (1993) Ecotoxicological relevance of atrazine in aquatic systems. Environ Toxicol Chem 12:1865–1881
Kazeto Y, Place AR, Trant JM (2004) Effects of endocrine disrupting chemicals on the expression of CYP19 genes in zebrafish (Danio rerio) juveniles. Aquat Toxicol 69:25–34
Kestemont P (1987) Etude du cycle reproducteur du goujon, Gobio gobio L.1. Variation saisonnière dans l’histologie de l’ovaire. J Appl Ichthyol 3:145–157
Kniewald J, Osredecki V, Gojmerac T, Zechner V, Kniewald Z (1995) Effect of s-triazine compounds on testosterone metabolism in the rat prostate. J Appl Toxicol 15:215–218
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mason JI, Carr BR, Murry BA (1987) Imidazole antimycotics: selective inhibitors of steroid aromatization and progesterone hydroxylation. Steroids 50:179–189
Mehats C, Tanguy G, Dallot E, Cabrol D, Ferre F, Leroy MJ (2001) Is up-regulation of phosphodiesterase 4 activity by PGE2 involved in the desensitization of beta-mimetics in late pregnancy human myometrium? J Clin Endocrinol Metab 86:5358–5365
Moore A, Waring CP (1998) Mechanistic effects of a triazine pesticide on reproductive endocrine function in mature male Atlantic salmon (Salmo salar L.). Pest Biochem Physiol 62:41–50
Morinaga H, Yanase T, Nomura M, Okabe T, Goto K, Harada N, Nawata H (2004) A benzimidazole fungicide, benomyl, and its metabolite, carbendazim, induce aromatase activity in a human ovarian granulose-like tumor cell line (KGN). Endocrinology 145:1860–1869
Noaksson E, Tjarnlund U, Bosveld ATC, Balk L (2001) Evidence for endocrine disruption in perch (Perca fluvatilis) and roach (Rutilus rutilus) in a remote Swedish lake in the vicinity of a public refuse dump. Appl Pharmacol 174:160–176
Roberge M, Hakk H, Larsen G (2004) Atrazine is a competitive inhibitor of phosphodiesterase but does not affect the estrogen receptor. Toxicol Lett 154:61–68
Sanderson JT, Seinen W, Giesy JP, Van den Berg M (2000) 2-Chloro-s-triazine herbicides induce aromatase (CYP19) activity in H295R human adrenocortical carcinoma cells: a novel mechanism for estrogenicity? Toxicol Sci 54:121–127
Sanderson JT, Letcher RJ, Heneweer M, Giesy JP, Van den Berg M (2001) Effects of chloro-s-triazine herbicides and metabolites on aromatase activity in various human cell lines and on vitellogenin production in male carp hepatocytes. Environ Health Perspect 109:1027–1031
Sanderson JT, Boerma J, Lansbergen GW, Van den Berg M (2002) Induction and inhibition of aromatase (CYP19) activity by various classes of pesticides in H295R human adrenocortical carcinoma cells. Toxicol Appl Pharmacol 182:44–54
Spanò L, Tyler CR, van Aerle R, Devos P, Mandiki SN, Silvestre F, Thome JP, Kestemont P (2004) Effects of atrazine on sex steroid dynamics, plasma vitellogenin concentration and gonad development in adult goldfish (Carassius auratus). Aquat Toxicol 66:369–379
Solomon KR, Baker DB, Richards RP, Dixon KR, Klaine SJ, La Point TW, Kendall RR, Weisskopf CP, Giddings JM, Giesy JP, Hall Jr LW, Williams WM (1996) Ecological risks assessment of atrazine in North America surface waters. Environ Toxicol Chem 15:31–76
Tennant MK, Hill DS, Eldridge JC, Wetzel LT, Breckenridge CB, Stevens JT (1994) Chloro-s-triazine antagonism of estrogen action: limited interaction with estrogen receptor binding. J Toxicol Environ Health 43:197–211
Vinggaard AM, Hnida C, Breinholt V, Larsen JC (2000) Screening of selected pesticides for inhibition of CYP19 aromatase activity in vitro. Toxicol In Vitro 14:227–234
Wetzel LT, Luempert LG, Breckenridge CB, Tisdel MO, Stevens JT, Thakur AK, Extrom PJ, Eldridge JC (1994) Chronic effects of atrazine on estrus and mammary tumor formation in female Sprague-Dawley and Fischer 344 rats. J Toxicol Environ Health 43:169–182
Wilhelms KW, Cutler SA, Proudman JA, Anderson LL, Scanes CG (2005) Atrazine and the hypothalamo-pituitary-gonadal axis in sexually maturing precocial birds: studies in male Japanese quail. Toxicol Sci 86:152–160
You L, Sar M, Bartolucci E, Ploch S, Whitt M (2001) Induction of hepatic aromatase by p,p′-DDE in adult male rats. Mol Cell Endocrinol 178:207–214
Acknowledgments
The authors are grateful to G. Trausch and A. Evrard for their technical help and M. Albert and A. Decreux for their contribution in the optimization of RNA extraction/PCR technique. The authors are also grateful to the Laboratory of Histology and Embryology of FUNDP for access to histological equipment. This study was performed with the financial support of the ‘Fonds de la Recherche Scientifique’ (FRS-FNRS contract 2.4.544.02F).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nadzialek, S., Spanò, L., Mandiki, S.N.M. et al. High doses of atrazine do not disrupt activity and expression of aromatase in female gonads of juvenile goldfish (Carassius auratus L.). Ecotoxicology 17, 464–470 (2008). https://doi.org/10.1007/s10646-008-0198-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-008-0198-9