Abstract
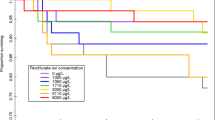
In October 2001 and March 2002, a field survey of central stonerollers (Campostoma anomalum) from perchlorate-contaminated streams in central Texas was conducted to assess thyroid endocrine disruption. A survey of adult male and female cricket frogs (Acris crepitans) was performed at the same site between 2001 and 2003. Perchlorate is an oxidizer primarily used in solid-fuel rockets, and many sites that processed or used perchlorate are now contaminated. Histological analysis revealed that the fish from contaminated sites had increased thyroid follicular hyperplasia, hypertrophy, and colloid depletion. Multivariate analysis was generally found to be more powerful than univariate analysis. Seasonal differences existed in the degree of thyroidal perturbation were discovered, and fish were generally less sensitive to thyroidal perturbations in March compared to October. Thyroidal histological indicators were also correlated to levels of perchlorate in the fish, water, and periphyton. Periphyton was frequently most strongly correlated to thyroidal indices, suggesting that exposure through the food chain may be of import. In addition, one of the presumed reference sites turned out to be contaminated with perchlorate, and this was reflected by thyroidal biomarkers before perchlorate was detected in the stream water or biota. There was no evidence of colloid depletion or hyperplasia in frogs from any of the sites, although frogs from two sites with greatest mean water perchlorate concentrations exhibited significantly greater follicle cell hypertrophy. Furthermore, there was a significant positive correlation between follicle cell height and mean water perchlorate concentrations for frogs collected from all sites. This is the first known published account of perchlorate-induced thyroid disruption in fish under field situations, only the second known published account for amphibians, and also points out the value of biomarkers for contaminant biomonitoring.









Similar content being viewed by others
References
Aas-Hansen O., Johnsen H.K., Vijayan M.M., Jorgensen E.H., (2003). Development of seawater tolerance and concurrent hormonal changes in fed and fasted Arctic charr at two temperature regimes Aquaculture 222: 135–48
Adams S.M., (2003). Establishing causality between environmental stressors and effects on aquatic ecosystems Hum. Ecol. Risk Assess. 9: 17–35
Anderson T.A., Wu T.H., (2002). Extraction, cleanup, and analysis of the perchlorate anion in tissue samples Bull. Environ. Contam. Toxicol. 68: 684–91
Anderson, T.A., Smith P.N., McMurry, S.T., Carr, J.A., Theodorakis, C.W., Jackson, W.A. and Dixon, K.R. (2004). Ecological risk assessment of ammonium perchlorate on fish, amphibian, and mammals in the Lake Belton and Lake Waco watersheds. An integrated laboratory and field investigation. In U.S. Army Corps of Engineers, Bosque and Leon River Watershed Study, Final Report. Fort Worth, TX: U.S. Army Corps of Engineers Fort Worth District
Bhattacharya L., (1995). Histological and histochemical alterations in the thyroid activity of endosulphan treated Oreochromis mossambicus J. Environ. Biol. 16: 347–51
Bradford, C.M., Carr, J.A., Rinchard, J., and Theodorakis, C. (2005) Perchlorate affects thyroid function in eastern mosquitofish (Gambusia holbrooki) at environmentally relevant concentrations. Eviron. Sci. Technol. 39, 5190-5
Brown S.B., Adams B.A., Cyr D.G., Eales J.G., (2004a). Contaminant effects on the teleost thyroid Environ. Toxicol. Chem. 23: 1680–701
Brown S.B., Evans R.E., Vandenbyllardt L., Finnson K.W., Palace V.P., Kane A.S., Yarechewski A.Y., Muir D.C.G., (2004b). Altered thyroid status in lake trout (Salvelinus namaycush). exposed to co-planar 3,3′,4,4′,5-pentachlorobiphenyl Aquat.Toxicol. 67: 75–85
Burkhead, N.M. (1980). Impact of commercial harvest on stoneroller populations. The life history of the stoneroller minnow Campostoma a. anomalum (Rafinesque) in five streams in east Tennessee. Master’s Thesis, University of Tennessee. Nashville, TN: Tennessee Wildlife Resources Agency
Carr J.A., Urquidi L.J., Goleman W.L., Hu F., Smith P.N., Theodorakis C.W., 2003. Ammonium perchlorate disruption of thyroid function in natural amphibian populations: Assessment and potential impact In: G. Linder, S. Krest, E.E. Little, (eds). Multiple Stressor Effects in Relation to Declining Amphibian Populations. ASTM STP 1443 West Conshohocken, PA ASTM International
Castonguay M., Cyr D.G., (1998). Effects on temperature on spontaneous and thyroxine-stimulated locomotor activity of Atlantic cod J. Fish Biol. 53: 303–13
Clark J.J., (2000). Toxicology of perchlorate In: Urbansky E.T., (eds). Perchlorate in the Environment New York Kluwer Academic/Plenum Publishers pp 15–29
Cooley H.M., Fisk A.T., Wiens S.C., Tomy G.T., Evans R.E., Muir D.C.G., (2001). Examination of the behavior and liver and thyroid histology of juvenile rainbow trout (Oncorhynchus mykiss). exposed to high dietary concentrations of C sub(10).-, C sub(11).-, C sub(12).- and C sub(14).-polychlorinated n-alkanes Aquat. Toxicol 54: 81–99
Crouch, N.T. (2003). Investigation of the effects of perchlorate on thyroid and reproductive system function in goldfish. Master’s Thesis. School of Forest Resources, Pennsylvania State University
Cyr D.G., Eales J.G., (1996). Interrelationships between thyroidal and reproductive endocrine systems in fish Rev. Fish Biol. Fisher 6: 165–200
Eales J.G., Brown S.B., (1993). Measurement and regulation of thyroidal status in teleost fish Rev. Fish Biol. Fisher 3: 299–347
Goleman W.L., Carr J.A., Anderson T.A., (2002a). Environmentally relevant concentrations of ammonium perchlorate inhibit thyroid function and alter sex ratios in developing Xenopus laevis Environ. Toxicol. Chem. 21: 424–30
Goleman W.L., Urquidi L.J., Anderson T.A., Smith E.E., Kendall R.J., Carr J.A., (2002b). Environmentally relevant concentrations of ammonium perchlorate inhibit development and metamorphosis in Xenopus laevis Environ. Toxicol. Chem. 21: 590–7
Greer M.A., Goodman G., Pleus R.C., Greer S.E., (2002). Health effects assessment for environmental perchlorate contamination, the dose response for inhibition of thyroid radioiodine uptake in human Environ. Health Perspect. 110: 927–37
Grinwis G.C.M., Besselink H.T., van den Brandhof E.J., Bulder A.S., Engelsma M.Y. Kuiper R.V., Wester P.W., Vaal M.A., Vethaak A.D., Vos J.G., (2000). Toxicity of TCDD in European flounder (Platichthys flesus) with emphasis on histopathology and cytochrome P450 1A induction in several organ systems Aquat. Toxicol. 50:387–401
Hollander M., Wolfe D.A., (1973). Nonparametric Statistical Methods New York John Wiley & Sons
Howdeshell K.L., (2002). A model of the development of the brain as a construct of the thyroid system Environ. Health Perspect. 110 Suppl 110(suppl 3): 337–48
Lanno R.P., Dixon D.G., (1994). Chronic toxicity of waterborne thiocyanate to the fathead minnow (Pimephales promelas), a partial life-cycle study Environ. Toxicol. Chem. 13: 1423–32
Lanno R.P., Dixon D.G., (1996). The comparative chronic toxicity of thiocyanate and cyanide to rainbow trout Aquat. Toxicol. 36: 177–87
Leatherland J.F., (1994). Reflections on the thyroidoology of fishes, from molecules to Humankind Guelph. Icthyol. Rev. 2: 3–67
MacKenzie D.S., VanPutte C.M., Leiner K.A., (1987). Nutrient regulation of endocrine function in fish Aquaculture 161: 3–25
MacKenzie D.S., Thomas P., Farrar S.M., (1989). Seasonal changes in thyroid and reproductive steroid hormones in female channel catfish (Ictalurus punctatus) in pond culture Aquaculture 78: 63–80
Manzon R.G., Holmes J.A., Youson J.H., (2001). Variable effects of giotrogens in inducing precocious metamorphosis in sea lampreys (Petromyzon marinus) J. Exp. Zool. 289: 290–303
Miranda L.A., Paz D.A., Dezi R.E., Pisano A., (1995). Immunocytochemical and morphometric study of TSH, PRL, GH, and ACTH cells in Bufo arenarum larvae with inhibited thyroid function Gen. Comp. Endocrino. 98: 166–76
Miranda L.A., Pisano A., Paz D.A., (1992). Effect of potassium perchlorate on thyroid activity of Bufo arenarum larvae Comunicaciones Biologicas 10: 125–35
Motzer W.E., (2001). Perchlorate, problem, detection, and solutions Environ. Forensics 2: 301–11
Nugegoda D., Wu W., Xu Y., Zhang J., Lichtmannegger J., Schramm K.-W., (2000). Thyroid hormones in fish exposed to PCDD/F and TCDD, from the Yangtze River region, China Organohalogen Compounds 49: 469–73
Palace V.P., Allen-Gil S.M., Brown S.B., Evans R.E., Metner D.A., Landers D.H., Curtis L.R., Klaverkamp J.F., Baron C.L., Lockhart W.L., (2001). Vitamin and thyroid status in arctic grayling (Thymallus arcticus) exposed to doses of 3,3′,4,4′-tetrachlorobiphenyl that induce the phase I enzyme system Chemosphere 45: 185–93
Park D., Minor M.D., Propper C.R., (2004). Toxic response of endosulfan to breeding and non-breeding female mosquitofish J. Environ. Biol. 25: 119–24
Patiño R., Wainscott M.R., Cruz-Li E.I., Balakrishnan S., McMurry C., Blazer V.S., Anderson T.A., (2003). Effects of ammonium perchlorate on the reproductive performance and thyroid condition of zebrafish Environ. Toxicol. Chem. 22: 1115–21
Pavlidis M., Dessypris A., Christofidis I., (1991). Seasonal fuctuations in plasma thyroid hormones, in two strains of rainbow trout (Oncorhynchus mykiss), during the first reproductive and second reproductive cycle – relation with their photoperiodically altered spawning time Aquaculture 99: 65–385
Peter S.M.C., Oommen O.V., (1993). Stiimulation of oxidative-metabolism by thyroid-hormones in propranolol alloxan-treated bony fish, Anabas testudineus (Bloch) J. Exp. Zool. 266: 85–91
Peter S.M.C., Lock R.A.C., Bonga S.E.W., (2000). Evidence for an osmoregulatory role of thyroid hormones in the freshwater mozambique tilapia Oreochromis mossambicus Gen. Comp. Endocrinol. 120: 157–67
Power D.M., Llewellyn L., Faustino M., Nowell M.A., Bjoernsson B.Th., Einarsdottir I.E., Canario A.V.M., Sweeney G.E., (2001). Thyroid hormones in growth and development of fish Comp. Biochem. Physiol. C 130: 447–59
Ram R.N., (1998). Carbofuran-induced histophysiological changes in thyroid of the teleost fish, Channa punctatus (Bloch) Ecotoxicol. Environ. Saf. 16: 106–13
Reddy P.K., Leatherland J.F., (2003). Influences of photoperiod and alternate days of feeding on plasma growth hormone and thyroid hormone levels in juvenile rainbow trout J. Fish Biol. 63: 197–212
Ricard A.C., Daniel C., Anderson P., Hontela A., (1998). Effects of subchronic exposure to cadmium chloride on endocrine and metabolic functions in rainbow trout Oncorhynchus mykiss Arch. Environ. Contam. Toxicol. 34: 377–81
Shi Y.B., (2000). Amphibian Metamorphosis: From Morphology to Molecular Biology Wiley-Liss New York, NY, USA
Smith P.N., Theodorakis C.W., Anderson T.A., Kendall R.J., (2001). Preliminary assessment of perchlorate in ecological receptors at the Longhorn Army Ammunition Plant (LHAAP), Karnack, Texas Ecotoxicology 10: 305–13
Sonstegard R.A., Leatherland J.F., (1984). Great Lakes coho salmon as an indicator organism for ecosystem health Mar. Environ. Res. 14: 480
Sparling D.W., Harvey G., Nzengung V., (2003). Interaction between perchlorates and iodine in the metamorphosis of Hyla versicolor In: G. Linder, S. Krest, E.E. Little, (eds). Multiple Stressor Effects in Relation to Declining Amphibian Populations. ASTM STP 1443 West Conshohocken, PA ASTM International
Stanbury J.B., Wyngaarden J.B., (1952). Effect of perchlorate on the human thyroid gland Metabolism 1: 533–9
Urbansky E.T., (Eds). (2000). Perchlorate in the Environment New York Kluwer Academic/Plenum Publishers
Urbansky E.T., (2002). Perchlorate as an environmental contaminant Environ. Sci. Pollut. Res. 9: 187–92
[USACE] U.S. Army Corps of Engineers (2004). Bosque and Leon River Watershed Study, Final Report Fort Worth, TX U.S. Army Corps of Engineers Fort Worth District
Wolff J., (1998). Perchlorate and the thyroid gland Pharmacol. Rev. 50: 89–105
York R.C., Brown W.R., Girard M.F., Dollarhide J.S., (2001). Two-generation reproduction study of ammonium perchlorate in drinking water in rats evaluates thyroid toxicity Int. J. Toxicol. 20: 183–97
Zhou T., John-Alder H.B., Weis J.S., Weis P., (1999a). Endocrine disruption: thyroid dysfunction in mummichogs (Fundulus heteroclitus) from a polluted habitat Mar. Environ. Res. 50:393–7
Zhou T., John-Alder H.B., Weis P., Weis J.S., (1999b). Thyroidal status of mummichogs (Fundulus heteroclitus) from a polluted versus a reference habitat Environ. Toxicol. Chem. 18: 2817–23
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Theodorakis, C.W., Rinchard, J., Carr, J.A. et al. Thyroid Endocrine Disruption in Stonerollers and Cricket Frogs from Perchlorate-Contaminated Streams in East-Central Texas. Ecotoxicology 15, 31–50 (2006). https://doi.org/10.1007/s10646-005-0040-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-005-0040-6