Summary
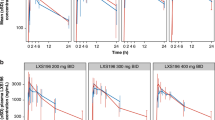
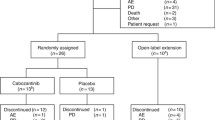
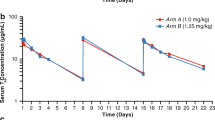
Background Endothelin B receptor (ETBR) is involved in melanoma pathogenesis and is overexpressed in metastatic melanoma. The antibody-drug conjugate DEDN6526A targets ETBR and is comprised of the humanized anti-ETBR monoclonal antibody conjugated to the anti-mitotic agent monomethyl auristatin E (MMAE). Methods This Phase I study evaluated the safety, pharmacokinetics, pharmacodynamics, and anti-tumor activity of DEDN6526A (0.3–2.8 mg/kg) given every 3 weeks (q3w) in patients with metastatic or unresectable cutaneous, mucosal, or uveal melanoma. Results Fifty-three patients received a median of 6 doses of DEDN6526A (range 1–49). The most common drug-related adverse events (>25% across dose levels) were fatigue, peripheral neuropathy, nausea, diarrhea, alopecia, and chills. Three patients in dose-escalation experienced a dose-limiting toxicity (infusion-related reaction, increased ALT/AST, and drug-induced liver injury). Based on cumulative safety data across all dose levels, the recommended Phase II dose (RP2D) for DEDN6526A was 2.4 mg/kg intravenous (IV) q3w. The pharmacokinetics of antibody-conjugated MMAE and total antibody were dose-proportional at doses ranging from 1.8–2.8 mg/kg. A trend toward faster clearance was observed at doses of 0.3–1.2 mg/kg. There were 6 partial responses (11%) in patients with metastatic cutaneous or mucosal melanoma, and 17 patients (32%) had prolonged stable disease ≥6 months. Responses were independent of BRAF mutation status but did correlate with ETBR expression. Conclusion DEDN6526A administered at the RP2D of 2.4 mg/kg q3w had an acceptable safety profile and showed evidence of anti-tumor activity in patients with cutaneous, mucosal, and uveal melanoma. ClinicalTrials.gov identifier: NCT01522664.


Similar content being viewed by others
References
Siegel RLMK, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7–30
Van Allen EM, Wagle N, Sucker A, Treacy DJ, Johannessen CM, Goetz EM, Place CS, Taylor-Weiner A, Whittaker S, Kryukov GV, Hodis E, Rosenberg M, McKenna A, Cibulskis K, Farlow D, Zimmer L, Hillen U, Gutzmer R, Goldinger SM, Ugurel S, Gogas HJ, Egberts F, Berking C, Trefzer U, Loquai C, Weide B, Hassel JC, Gabriel SB, Carter SL, Getz G, Garraway LA, Schadendorf D, Dermatologic Cooperative Oncology Group of Germany (DeCOG) (2014) The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov 4:94–109
Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A (2017) Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168:707–723
Wahid M, Jawed A, Mandal RK, Dar SA, Akhter N, Somvanshi P, Khan F, Lohani M, Areeshi MY, Haque S (2018) Recent developments and obstacles in the treatment of melanoma with BRAF and MEK inhibitors. Crit Rev Oncol Hematol 125:84–88
Bagnato A, Rosanò L, Spinella F, Di Castro V, Tecce R, Natali PG (2004) Endothelin B receptor blockade inhibits dynamics of cell interactions and communications in melanoma cell progression. Cancer Res 64:1436–1443
Lappano R, Maggiolini M (2011) G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov 10:47–60
Saldana-Caboverde A, Kos L (2010) Roles of endothelin signaling in melanocyte development and melanoma. Pigment Cell Melanoma Res 23:160–170
Asundi J, Reed C, Arca J, McCutcheon K, Ferrando R, Clark S, Luis E, Tien J, Firestein R, Polakis P (2011) An antibody-drug conjugate targeting the endothelin B receptor for the treatment of melanoma. Clin Cancer Res 17:965–975
Demunter A, De Wolf-Peeters C, Degreef H, Stas M, van den Oord JJ (2001) Expression of the endothelin-B receptor in pigment cell lesions of the skin. Evidence for its role as tumor progression marker in malignant melanoma. Virchows Arch 438:485–491
Nelson J, Bagnato A, Battistini B, Nisen P (2003) The endothelin axis: emerging role in cancer. Nat Rev Cancer 3:110–106
Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, Radmacher M, Simon R, Yakhini Z, Ben-Dor A, Sampas N, Dougherty E, Wang E, Marincola F, Gooden C, Lueders J, Glatfelter A, Pollock P, Carpten J, Gillanders E, Leja D, Dietrich K, Beaudry C, Berens M, Alberts D, Sondak V (2000) Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature 406:536–540
Smith SL, Damato BE, Scholes AG, Nunn J, Field JK, Heighway J (2002) Decreased endothelin receptor B expression in large primary uveal melanomas is associate with early clinical metastasis and short survival. Br J Cancer 87:1308–1313
Bai R, Pettit GR, Hamel E (1990) Binding of dolastatin 10 to tubulin at a distinct site for peptide antimitotic agents near the exchangeable nucleotide and vinca alkaloid sites. J Biol Chem 265:17141–17149
Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, DeBlanc RL, Gearing RP, Bovee TD, Siegall CB, Francisco JA, Wahl AF, Meyer DL, Senter PD (2003) Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol 21:778–84, 784
Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, Rejniak SX, Gordon KA, DeBlanc R, Toki BE, Law CL, Doronina SO, Siegall CB, Senter PD, Wahl AF (2003) cAC10-vcMMAE, an anti-CD30−monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood 102:1458–1465
Okeley NM, Miyamoto JB, Zhang X, Sanderson RJ, Benjamin DR, Sievers EL, Senter PD, Alley SC (2010) Intracellular activation of SGN-35, a potent anti-CD30 antibody-drug conjugate. Clin Cancer Res 16:888–97, 897
Abe Y, Nakayama K, Yamanaka A, Sakurai T, Goto K (2000) Subtype-specific trafficking of endothelin receptors. J Biol Chem 275:8664–8671
Meulendijks D, Jacob W, Voest EE, Mau-Sorensen M, Martinez-Garcia M, Taus A, Fleitas T, Cervantes A, Lolkema MP, Langenberg MHG, De Jonge MJ, Sleijfer S, Han JY, Calles A, Felip E, Kim SW, Schellens JHM, Wilson S, Thomas M, Ceppi M, Meneses-Lorente G, James I, Vega-Harring S, Dua R, Nguyen M, Steiner L, Adessi C, Michielin F, Bossenmaier B, Weisser M, Lassen UN (2017) Phase Ib study of lumretuzumab plus cetuximab or erlotinib in solid tumor patients and evaluation of HER3 and heregulin as potential biomarkers of clinical activity. Clin Cancer Res 23:5406–5415
Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ, Luo Y (2012) RNAscope. a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues J Mol Diagn 14:22–29
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50:122S–150S
Van de Donk NW, Dhimolea E (2012) Brentuximab vedotin. MAbs 4:458–465
Palanca-Wessels MC, Czuczman M, Salles G, Assouline S, Sehn LH, Flinn I, Patel MR, Sangha R, Hagenbeek A, Advani R, Tilly H, Casasnovas O, Press OW, Yalamanchili S, Kahn R, Dere RC, Lu D, Jones S, Jones C, Chu YW, Morschhauser F (2015) Safety and activity of the anti-CD79B antibody-drug conjugate polatuzumab vedotin in relapsed or refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukaemia: a phase 1 study. Lancet Oncol 16:704–715
Liu JF, Moore KN, Birrer MJ, Berlin S, Matulonis UA, Infante JR, Wolpin B, Poon KA, Firestein R, Xu J, Kahn R, Wang Y, Wood K, Darbonne WC, Lackner MR, Kelley SK, Lu X, Choi YJ, Maslyar D, Humke EW, Burris HA (2016) Phase I study of safety and pharmacokinetics of the anti-MUC16 antibody-drug conjugate DMUC5754A in patients with platinum-resistant ovarian cancer or unresectable pancreatic cancer. Ann Oncol 27:2124–2130
Advani RH, Lebovic D, Chen A, Brunvand M, Goy A, Chang JE, Hochberg E, Yalamanchili S, Kahn R, Lu D, Agarwal P, Dere RC, Hsieh HJ, Jones S, Chu YW, Cheson BD (2017) Phase I study of the anti-CD22 antibody-drug conjugate pinatuzumab vedotin with/without rituximab in patients with relapsed/refractory b-cell non-hodgkin lymphoma. Clin Cancer Res 23:1167–1176
Acknowledgements
We thank the patients and their families who took part in the study, as well as the staff, research coordinators, and investigators at each participating institution. Writing assistance provided by Genentech, Inc.
Funding
This work was supported by Genentech, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SS: Consultant/advisory board member for Roche-Genentech, Novartis, Astra-Zeneca, Bristol Meyers Squibb, Merck Sharp and Dolme and Amgen. Research grant funding from Amgen, Bristol Meyers Squibb, Merck Sharp and Dolme, Merck and Genentech.
CM: None.
PL: None.
MP: Speakers bureau/advisory boards for Pfizer, Exelixis, Pharmacyclics, Celgene, and Bayer.
OK: Employee of Genentech, Inc., shareholder of F. Hoffmann La Roche, Ltd.
CL: Employee of Genentech, Inc., shareholder of F. Hoffmann La Roche, Ltd.
WF: Employee of Genentech, Inc., shareholder of F. Hoffmann La Roche, Ltd.
RY: Employee of Genentech, Inc., shareholder of F. Hoffmann La Roche, Ltd.
FB: Employee of Genentech, Inc., shareholder of F. Hoffmann La Roche, Ltd.
RJH: Advisory board member and stockholder of Telix Pharmaceuticals. Consultant for Endocyte. Research grant funding from Ipsen and ITG.
DN: Employee of Genentech, Inc., shareholder of F. Hoffmann La Roche, Ltd.
VL: Employee of Genentech, Inc., shareholder of F. Hoffmann La Roche, Ltd.
RM: Employee of Genentech, Inc., shareholder of F. Hoffmann La Roche, Ltd.
OH: Consulting for Roche, Amgen, Novartis, BMS, Merck. Speaker for Genentech, BMS, Novartis, Amgen, Sanofi, Array. Contracted research for institution from Roche-Genentech, Amgen, Arcus, Astellas, AstraZeneca, BMS, Celldex, Cytomx, GSK, Immunocore, Incyte, Iovance, Merck, Merck Serono, MedImmune, NextCure, Novartis, Parker, Pfizer, Polynoma, Regeneron.
JI: Consulting for Armo Biosciences, Bio Med Valley Discoveries. Employee of Janssen Pharmaceuticals.
Ethical approval
This study was conducted at 6 institutions in the United States and Australia in accordance with the International Conference on Harmonization E6 Guidelines and the principles of Good Clinical Practice. The study was approved by regulatory and ethics committees at each institution and was registered at Clinicaltrials.gov, NCT01522664. The study was sponsored by Genentech, Inc.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Investigational New Drugs
https://www.springer.com/medicine/oncology/journal/10637
Electronic supplementary material
ESM 1
(DOCX 328 kb)
Rights and permissions
About this article
Cite this article
Sandhu, S., McNeil, C.M., LoRusso, P. et al. Phase I study of the anti-endothelin B receptor antibody-drug conjugate DEDN6526A in patients with metastatic or unresectable cutaneous, mucosal, or uveal melanoma. Invest New Drugs 38, 844–854 (2020). https://doi.org/10.1007/s10637-019-00832-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-019-00832-1