Summary
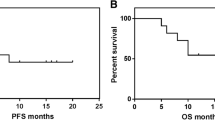
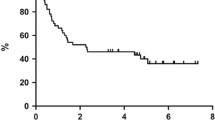
Metronomic-chemotherapy (M-CHT) has been rarely assessed in non-Hodgkin-lymphoma (NHL). Therefore, in 2011 we started experimenting a new all-oral M-CHT schedule termed DEVEC (Deltacortene®, etoposide, vinorelbine, cyclophosphamide, +/-Rituximab) in diffuse-large-B-cell lymphoma (DLBCL) patients. Methods Patients with stage Ib-IV were enrolled as follows: 1) treatment-naïve, frail ≥65y, or unfit ≥85y; and 2) relapsed/refractory (R/R) ≥55y. Data were prospectively collected from six Italian centres and compared for efficacy to two reference groups, treated with established iv Rituximab-CHT in 1st and 2nd line respectively. Results from April-2011 to March-2018, 17/51(33%) naïve, 21/51(41%) refractory and 13/51(25.5%) relapsed patients started DEVEC; 39/51(76.5%) were de-novo DLBCL; 10/51(19.6%) transformed-DLBCL and 2/51(3.9%) unclassifiable-DLBCL/classical-Hodgkin-lymphoma. The median age was 85y (range=77-93) and 78y (range=57-91) in naïve and R/R respectively and overall the DEVEC patients had very poor features compared to the reference. The rate of grade≥3 haematological-AEs was 43%(95CI=29-58%): G3-neutropenia was the most frequent; grade≥3 extra-haematological-AEs was 13.7% (95%CI=5.4-25.9%), the most frequent was infection. One-year OS and PFS were 67% and 61% for naive, 60% and 50% for reference-naïve respectively; Cox proportional hazard ratio (Cox-PH-ratio) for OS and PFS were 0.69 (95%CI=0.27-1.76;p=.441) and 0.68 (95%CI=0.28-1.62;p=.381) respectively. One-year OS and PFS were 48% and 39% in the R/R, 36% and 17% in the reference-R/R respectively; Cox-PH-ratio for OS and PFS, were 0.76 (95%CI=0.42-1.40; p=.386) and 0.48 (95%CI=0.28-0.82; p=.007) respectively. Conclusion The favourable activity of DEVEC compared to a real-life series and the convenience of an oral administration, may possibly lay the groundwork for a paradigm-shift in the treatment of elderly DLBCL.



Similar content being viewed by others
References
Tucci A, Martelli M, Rigacci L, Riccomagno P, Cabras MG, Salvi F et al (2015) Comprehensive geriatric assessment is an essential tool to support treatment decisions in elderly patients with diffuse large B-cell lymphoma: a prospective multicenter evaluation in 173 patients by the Lymphoma Italian Foundation (FIL). Leuk Lymphoma 56(4):921–926
Dinmohamed AG, Issa DE, Van der Poel MWM, Schouten HC, Lugtenburg PJ, Chamuleau MED et al (2017) Treatment and relative survival in very elderly patients with DLBCL in The Netherlands: a population-based study, 1989 to 2015. Blood Advances 1(21):1839–1841
Buske C, Hutchings M, Ladetto M, Goede V, Mey U, Soubeyran P (2018) ESMO Lymphoma Consensus Conference Panel Members. ESMO Consensus Conference on malignant lymphoma: general perspectives and recommendations for the clinical management of the elderly patient with malignant lymphoma. Ann Oncol 29(3):544–562
Peyrade F, Jardin F, Thieblemont C, Thyss A, Emile JF, Castaigne S et al (2011) Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol 12(5):460–468
Peyrade F, Bologna S, Delwail V, Emile JF, Pascal L, Fermé C et al (2017) Combination of ofatumumab and reduced-dose CHOP for diffuse large B-cell lymphomas in patients aged 80 years or older: an open-label, multicentre, single-arm, phase 2 trial from the LYSA group. Lancet Haematology 4:e46–e55
Merli F, Luminari S, Rossi G, Mammi C, Marcheselli L, Ferrari A et al (2014) Outcome of frail elderly patients with diffuse large B-cell lymphoma prospectively identified by Comprehensive Geriatric Assessment: results from a study of the Fondazione Italiana Linfomi. Leuk Lymphoma 55(1):38–43
Lin RJ, Behera M, Diefenbach CS (2017) Flowers CR Role of anthracycline and comprehensive geriatric assessment for elderly patients with diffuse large B-cell lymphoma. Blood. 130(20):2180–2185
Storti S, Spina M, Pesce EA, Salvi F, Merli M, Ruffini A et al (2018) Rituximab plus bendamustine as front-line treatment in frail elderly (>70 years) patients with diffuse large B-cell non-Hodgkin lymphoma: a phase II multicenter study of the Fondazione Italiana Linfomi. Haematologica. 103(8):1345–1350
Fields PA, Townsend W, Webb A, Counsell N, Pocock C, Smith P et al (2014) De novo treatment of diffuse large B-cell lymphoma with rituximab, cyclophosphamide, vincristine, gemcitabine, and prednisolone in patients with cardiac comorbidity: a United Kingdom National Cancer Research Institute trial. J Clin Oncol 32(4):282–287
Luminari S, Viel E, Ferreri AJM, Zaja F, Chimienti E, Musuraca G et al (2018) Non pegylated liposomal doxorubicin combination regimen in patients with diffuse large B-cell lymphoma and cardiac comorbidity. Results of the HEART01 phase II trial conducted by the Fondazione Italiana Linfomi. Hematol Oncol 36(1):68–75
Maybury B, Kimpton G, Otton S (2018) A retrospective multicentre study of COCKLE, an oral chemotherapy regimen, as palliative treatment for high grade lymphoma. British J Haematology. https://doi.org/10.1111/bjh.15637
Rashidi A, Oak E, Carson KR, Wagner-Johnston ND, Kreisel F, Bartlett NL (2016) Outcomes with R-CEOP for R-CHOP-ineligible patients with diffuse large B-cell lymphoma are highly dependent on cell of origin defined by Hans criteria. Leuk Lymphoma 57(5):1191–1193
Spina M, Balzarotti M, Uziel L, Ferreri AJ, Fratino L, Magagnoli M et al (2012) Modulated chemotherapy according to modified comprehensive geriatric assessment in 100 consecutive elderly patients with diffuse large B-cell lymphoma. Oncologist 17(6):838–846
Shen QD, Zhu HY, Wang L, Fan L, Liang JH, Cao L et al (2018) Gemcitabine-oxaliplatin plus rituximab (R-GemOx) as first-line treatment in elderly patients with diffuse large B-cell lymphoma: a single-arm, open-label, phase 2 trial. Lancet Haematology 5:e261–e269
Eyre TA, Salisbury R, Eyre DW, Watson C, Collins GP, Hatton CS et al (2016) Results of a large retrospective analysis of the effect of intended dose intensity of R-CHOP on outcome in a cohort of consecutive, unselected elderly patients with de novo diffuse large B cell lymphoma. Br J Haematol 173(3):487–491
Kumar A, Fraz MA, Usman M, Malik SU, Ijaz A, Durer C, Durer S, Tariq MJ, Khan AY, Qureshi A, Faridi W, Nasar A, Anwer F (2018 Sep 1) Treating Diffuse Large B Cell Lymphomain the Very Old or Frail Patients. Curr Treat Options in Oncol 19(10):50
Ohmachi K, Niitsu N, Uchida T, Kim SJ, Ando K, Takahashi N et al (2013) Multicenter phase II study of bendamustine plus rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 31(17):2103–2109
Arcari A, Chiappella A, Spina M, Zanlari L, Bernuzzi P, Valenti V et al (2016) Safety and efficacy of rituximab plus bendamustine in relapsed or refractory diffuse large B-cell lymphoma patients: an Italian retrospective multicenter study. Leuk Lymphoma 57(8):1823–1830
Corazzelli G, Capobianco G, Arcamone M, Ballerini PF, Iannitto E, Russo F et al (2009) Long-term results of gemcitabine plus oxaliplatin with and without rituximab as salvage treatment for transplant-ineligible patients with refractory/relapsing B-cell lymphoma. Cancer Chemother Pharmacol 64(5):907–916
Machover D, Delmas-Marsalet B, Misra SC, Gumus Y, Goldschmidt E (2001 Oct) Schilf A, et al Dexamethasone, high-dose cytarabine, and oxaliplatin (DHAOx) as salvage treatment for patients with initially refractory or relapsed non-Hodgkin's lymphoma. Ann Oncol 12(10):1439–1443
Bocci G, Kerbel R (2016) Pharmacokinetics of metronomic chemotherapy: a neglected but crucial aspect. Nat Rev Clin Oncol 13(11):659–673
Natale G, Bocci G (2018) Does metronomic chemotherapy induce tumor angiogenic dormancy? A review of available preclinical and clinical data. Cancer Lett 432:28–37
Coleman M, Martin P, Ruan J, Furman R, Niesvizky R, Elstrom R et al (2008) Prednisone, etoposide, procarbazine, and cyclophosphamide (PEP-C) oral combination chemotherapy regimen for recurring/refractory lymphoma: low-dose metronomic, multidrug therapy. Cancer. 112(12):2228–2232
Zeng J, Yang L, Huang F, Hong T, He Z, Lei J et al (2016) The metronomic therapy with prednisone, etoposide, and cyclophosphamide reduces the serum levels of VEGF and circulating endothelial cells and improves response rates and progression-free survival in patients with relapsed or refractory non-Hodgkin's lymphoma. Cancer Chemother Pharmacol 78(4):01–08
Schelker RC, Herr W, Reichle A, Vogelhuber M (2018) Low-dose trofosfamide plus rituximab is an effective and safe treatment for diffuse large B-cell lymphoma of the elderly: a single center experience. BMC Cancer 18(1):1000
Witte HM, Riecke A, Mayer T, Bartscht T, Rades D (2019 Jan) Lehnert H et alTrofosfamide in the treatment of elderly or frailpatients with diffuse large B-cell lymphoma. J Cancer Res Clin Oncol 145(1):129–136
Thompson DS, Hainsworth JD, Hande KR, Holzmer MC, Greco FA (1993) Prolonged administration of low-dose, infusional etoposide in patients with etoposide-sensitive neoplasms: a phase I/II study. J Clin Oncol
Balzarotti M, Santoro A, Tondini C, Fornier M, Bonadonna G (1996 Nov) Activity of singleagent vinorelbine in pretreated non-Hodgkin's lymphoma. Ann Oncol 7(9):970–972
Rule S, Tighe M, Davies S, Johnson S (1998 Sep) Vinorelbine in the treatment of lymphoma. Hematol Oncol 16(3):101–105
Man S, Bocci G, Francia G, Green SK, Jothy S (2002 May 15) Hanahan D et al Antitumor effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res 62(10):2731–2735
Bertolini F, Paul S, Mancuso P, Monestiroli S, Gobbi A, Shaked Y, Kerbel RS (2003 Aug 1) Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effectson the mobilization and viability of circulating endothelial progenitor cells. Cancer Res 63(15):4342–4346
Rozados VR, Sánchez AM, Gervasoni SI, Berra HH, Matar P, Graciela SO (2004 Oct) Metronomic therapy with cyclophosphamide induces rat lymphoma and sarcomaregression, and is devoid of toxicity. Ann Oncol 15(10):1543–1550
Briasoulis E, Aravantinos G, Kouvatseas G, Pappas P, Biziota E, Sainis, I, et al. Dose selection trial of metronomic oral vinorelbine monotherapy in patients with metastatic cancer: a hellenic cooperative oncology group clinical translational study BMC Cancer 2013;13:263.
Minard-Colin V, Ichante JL, Nguyen L, Paci A, Orbach D, Bergeron C et al (2012 Oct) Phase II study of vinorelbine and continuous low doses cyclophosphamide in children and young adults with a relapsed or refractory malignant solid tumour: good tolerance profile and efficacy in rhabdomyosarcoma--a report from the Société Française des Cancers et leucémies del'Enfant et de l'adolescent (SFCE). Eur J Cancer 48(15):2409–2416
Mavroeidis L, Sheldon H, Briasoulis E, Marselos M, Pappas P, Harris AL (2015) Metronomic vinorelbine: Anti-angiogenic activity in vitro in normoxic and severe hypoxic conditions, and severe hypoxia-induced resistance to its anti-proliferative effect with reversal by Akt inhibition. Int J Oncol
Launay S, Sabatier R, Brunelle S, Esterni B, Tarpin C, Viret Fet al . METRO1: A Phase I Study of Metronomic Chemotherapy in Adults with Advanced Refractory Solid Tumors. Anticancer Res 2016 Jan;36(1):293-299.
Orecchioni S, Talarico G, Labanca V, Calleri A, Mancuso P, Bertolini F (2018 May) Vinorelbine, cyclophosphamide and 5-FU effects on the circulating andi ntratumoural landscape of immune cells improve anti-PD-L1 efficacy in preclinical models of breast cancer and lymphoma. Br J Cancer 118(10):1329–1336. https://doi.org/10.1038/s41416-018-0076-z
Orlandi P, Di Desidero T, Salvia G, Muscatello B, Francia G, Bocci G (2018) Metronomic vinorelbine is directly active on Non Small Cell Lung Cancer cells and sensitizes the EGFRL858R/T790M cells to reversible EGFR tyrosine kinase inhibitors. Biochem Pharmacol 152:327–337
Cox MC, Musuraca G, Battistini R, Casaroli I, Zoli V, Anticoli-Borza P et al (2018) Aggressive lymphomas of the elderly: the DEVEC metronomic chemotherapy schedule fits the unfit. Br J Haematol 183(5):819–822
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R et al (2016) The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 127(20):2375–2390
Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800-1808. Blood. 2018 Feb 1;131(5):587-588
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25(5):579–586
Cox MC, Di Napoli A, Scarpino S, Salerno G, Tatarelli C, Talerico C et al (2014) Clinicopathologic characterization of diffuse-large-B-cell lymphoma with an associated serum monoclonal IgM component. PLoS One 9:e93903
Zinzani PL, Pellegrini C, Argnani L, Broccoli A (2016) Prolonged disease-free survival in elderly relapsed diffuse large B-cell lymphoma patients treated with lenalidomide plus rituximab. Haematologica. 101:e385–e386. https://doi.org/10.3324/haematol.2016.147256
Houot R, Cartron G, Bijou F, de Guibert S, Salles GA., Fruchart C, et al. Obinutuzumab plus Lenalidomide (GALEN) for the treatment of relapse/refractory aggressive lymphoma: a phase II LYSA study. Leukemia. 2018; https://doi.org/10.1038/s41375-018-0282-y.
Di Desidero T, Derosa L, Galli L, Orlandi P, Fontana A, Fioravanti A et al (2016) Clinical, pharmacodynamic and pharmacokinetic results of a prospective phase II study on oral metronomic vinorelbine and dexamethasone in castration-resistant prostate cancer patients. Investig New Drugs 34(6):760–770
NL Berinstein, AJ Grillo-López, CA White, et al: Association of serum rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin's lymphoma Ann Oncol 9: 995– 1001,1998
Rossi D, Diop F, Spaccarotella E, Monti S, Zanni M, Rasi S et al (2017) Diffuse large B-cell lymphoma genotyping on the liquid biopsy. Blood. 129(14):1947–1957
Park SI, Grover NS, Olajide O, Asch AS, Wall JG, Richards KL et al. A phase II trial of bendamustine in combination with rituximab in older patients with previously untreated diffuse 10.1111/bjh.14232. Epub 2016 Jul 22. PubMed PMID: 27448091; PubMed Central PMCID:
Ciccolini J, Barbolosi D, Meille C, Lombard A, Serdjebi C, Giacometti S et al (2017) Pharmacokinetics and Pharmacodynamics-Based Mathematical Modeling Identifies an Optimal Protocol for Metronomic Chemotherapy. Cancer Res 77(17):4723–4733
Kerbel RS (2017) Shaked Y The potential clinical promise of 'multimodality' metronomic chemotherapy revealed by preclinical studies of metastatic disease. Cancer Lett 400:293–304
Cerrito MG, De Giorgi M, Pelizzoni D, Bonomo SM, Digiacomo N, Scagliotti A et al (2018) Metronomic combination of Vinorelbine and 5Fluorouracil is able to inhibit triple-negative breast cancer cells. Results from the proof-of-concept VICTOR-0 study. Oncotarget. 9(44):27448–27459
André N, Tsai K, Carré M, Pasquier E (2017 May) Metronomic Chemotherapy: Direct Targeting of Cancer Cells after all? Trends Cancer 3(5):319–325
Buckstein R, Kerbel RS, Shaked Y, Nayar R, Foden C, Turner R et al (2006) High-Dose celecoxib and metronomic "low-dose" cyclophosphamide is an effective and safe therapy in patients with relapsed and refractory aggressive histology non-Hodgkin's lymphoma. Clin Cancer Res 12(17):5190–5198
Acknowledgments
M Christina Cox : performed the research, designed the research study, analysed the data, wrote the paper.
Sabrina Pelliccia: performed the research, analysed the data, wrote the paper
Luigi Marcheselli: designed the research study, analysed the data, wrote the paper
Roberta Battistini: performed the research, wrote the paper
Annalisa Arcari: performed the research, designed the research study, wrote the paper
Paola Anticoli Borza: performed the research, wrote the paper
Caterina Patti: performed the research, wrote the paper
Ivana Casaroli: performed the research, wrote the paper
Francesca di Landro: performed the research, wrote the paper
Arianna Di Napoli: performed the research, wrote the paper wrote the paper
Francesca Fabbri: performed the research, wrote the paper
Matteo Caridi: analysed the data, wrote the paper
Agostino Tafuri: performed the research, wrote the paper
Guido Bocci: designed the research study, analysed the data, wrote the paper
Gerardo Musuraca: performed the research, designed the research study, analysed the data, wrote the paper
Funding
The work was supported by the Department of Oncological science, Azienda Ospedaliera-Universitaria Sant’Andrea, Rome, Italy
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Author M Christina Cox declares that she has no conflict of interest;
Author Sabrina Pelliccia, declares that she has no conflict of interest,
Author Luigi Marcheselli, declares that he has no conflict of interest;
Author Roberta Battistini, declares that she has no conflict of interest;
Author Annalisa Arcari, declares that she has no conflict of interest;
Author Paola Anticoli Borza, declares that she has no conflict of interest;
Author Caterina Patti , declares that she has no conflict of interest;
Author Ivana Casaroli , declares that she has no conflict of interest;
Author Francesca di Landro , declares that she has no conflict of interest;
Author Arianna Di Napoli, declares that she has no conflict of interest;
Author Francesca Fabbri, declares that she has no conflict of interest;
Author Matteo Caridi, declares that he has no conflict of interest;
Author Agostino Tafuri , declares that he has no conflict of interest;
Author Guido Bocci , declares that he has no conflict of interest;
Author Gerardo Musuraca,, declares that he has no conflict of interest;
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Sapienza ethics committee (EC approval n° 4640).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cox, M.C., Pelliccia, S., Marcheselli, L. et al. The metronomic all-oral DEVEC is an effective schedule in elderly patients with diffuse large b-cell lymphoma. Invest New Drugs 37, 548–558 (2019). https://doi.org/10.1007/s10637-019-00769-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-019-00769-5