Summary
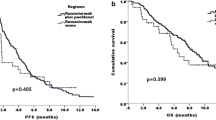
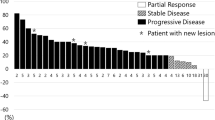
Purpose Few data described the activity of chemotherapy after ramucirumab plus paclitaxel progression in metastatic gastric cancer patients. The aim of this phase II study is to assess the efficacy and safety of the FOLFIRI regimen as a third-line of treatment. Methods The study enrolled patients with histologically proven metastatic gastric cancer or gastroesophageal junction carcinoma whose disease had progressed after ramucirumab-based second line of treatment. Treatment consisted of biweekly irinotecan 150 mg/m2 as a 1-h infusion on day 1, folinic acid 100 mg/m2 intravenously on days 1–2, and 5-fluorouracil as a 400 mg/m2 bolus and then 600 mg/m2 continuous infusion over 22 h on days 1–2. Primary end-point was tumor response rate (confirmed complete and partial response). Results Twenty-six patients were enrolled. Overall response rate and disease control rate were 11.5% and 38.5%. The median progression free survival (PFS) was 52 days (95% CI:42–74), and the median overall survival was 117 days (95% CI: 94–154). no unexpected adverse events have been observed. A longer PFS and OS were observed in patients who had achieved PFS ≥ 3 months during prior ramucirumab treatment. Conclusions Our findings suggest a poor efficacy of the FOLFIRI regimen in metastatic gastric or gastroesophageal junction cancer patients whose disease progressed during a ramucirumab-based second line of treatment. However, FOLFIRI could be an option for patients who responded to prior ramucirumab.



Similar content being viewed by others
References
De Manzoni G, Marrelli D, Baiocchi GL, Morgagni P, Saragoni L, Degiuli M et al (2017 Jan) The Italian research Group for Gastric Cancer (GIRCG) guidelines for gastric cancer staging and treatment: 2015. Gastric Cancer 20(1):20–30
Marano L, Polom K, Patriti A, Roviello G, Falco G, Stracqualursi A, et al (2015) Surgical management of advanced gastric cancer: An evolving issue. Eur J Surg Oncol. Nov 14.
Marrelli D, Polom K, Pascale V, Vindigni C, Piagnerelli R, De Franco L, Ferrara F, Roviello G, Garosi L, Petrioli R, Roviello F (2016 Mar) Strong prognostic value of microsatellite instability in intestinal type non-cardia gastric Cancer. Ann Surg Oncol 23(3):943–950
Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J (2014) REGARD Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. Jan 4 383(9911):31–39
Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D et al (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15(11):1224–1235
Kang JH, Lee SI, Lim DH, Park KW, Oh SY, Kwon HC et al (2012) Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive carealone. Clin Oncol 30(13):1513–1518
Kang EJ, Im SA, Oh DY, Han SW, Kim JS, Choi IS et al (2013) Irinotecan combined with 5-fluorouracil and leucovorin third-line chemotherapy afterfailure of fluoropyrimidine, platinum, and taxane in gastric cancer: treatment outcomes and a prognostic model to predict survival. Gastric Cancer 16(4):581–589
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009 Jan) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10
Chana WL, Yuen KK, Siu S, Lamb K, Kwong DL (2017) Third-line systemic treatment versus best supportive care for advanced/metastatic gastric cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol 116:68–81
Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y et al (2016) Randomized, double-blind,placebo-controlled phase III trial of apatinib in patients withchemotherapy-refractory advanced or metastatic adenocarcinoma of thestomach or gastroesophageal junction. J Clin Oncol 34(13):1448–1454
Petrioli R, Roviello G, Zanotti L, Roviello F, Polom K, Bottini A, Marano L, Francini E, Marrelli D, Generali D (2016 Jun) Epirubicin-based compared with docetaxel-based chemotherapy for advanced gastric carcinoma: a systematic review and meta-analysis. Crit Rev Oncol Hematol 102:82–88
Takahari D (2017 May) Second-line chemotherapy for patients with advanced gastric cancer. Gastric Cancer 20(3):395–406. https://doi.org/10.1007/s10120-017-0707-8 Epub 2017 Mar 4
De Vita F, Di Martino N, Fabozzi A, Laterza MM, Ventriglia J, Savastano B, Petrillo A, Gambardella V, Sforza V, Marano L, Auricchio A, Galizia G, Ciardiello F, Orditura M (2014 Oct 28) Clinical management of advanced gastric cancer: the role of new molecular drugs. World J Gastroenterol 20(40):14537–14558
Lordick F, Allum W, Carneiro F, Mitry E, Tabernero J, Tan P et al (2014 Jul) Unmet needs and challenges in gastric cancer: the way forward. Cancer Treat Rev 40(6):692–700
Yu Zheng MM, Xu-Qing Zhu BM, XiaoGang Ren MM (2017) Third-line chemotherapy in advanced gastric cancer A systematic review and meta-analysis. Medicine 96:24(e6884)
Erdem GU, Bozkaya Y, Ozdemir NY, Demirci NS, Yazici O, Zengin N (2018) 5-fluorouracil, leucovorin, and irinotecan (FOLFIRI) as a third-line chemotherapy treatment in metastatic gastric cancer, after failure of fluoropyrimidine, platinum, anthracycline, and taxane. Bosn J Basic Med Sci 20;18(2):170–177
Roviello G, Ravelli A, Fiaschi AI et al (2016) Apatinib for the treatment of gastric cancer. Expert Review of Gastroenterology & Hepatology 10(8):887–892
Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y et al (2013) Apatinib forchemotherapy-refractory advanced metastatic gastric cancer: results from arandomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 31(26):3219–3225
Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung HC, Pan HM et al (2013) Everolimus for previously treated advanced gastric cancer: results of therandomized double-blind phase III GRANITE-1 study. J Clin Oncol 31(31):3935–3943
Pavlakis N, Sjoquist KM, Tsobanis E, Martin AJ, Kang Y-K, Bang J, et al (2015) INTEGRATE: a randomized, phase II, double-blind, placebo-controlled study ofregorafenib in refractory advanced oesophagogastric cancer (AOGC): a studyby the Australasian Gastrointestinal Trials Group (AGITG)-final overall andsubgroup results. J. Clin. Oncol., Conference: 2015 Annual Meeting of theAmerican Society of Clinical Oncology, ASCO Chicago, IL United States. Conference Start: 20150529 Conference End: 20150602. ConferencePublication: (var.pagings). 33 (15 SUPPL. 1) (no pagination), 2015. Date ofPublication: 20 May 2015
Yee NS (2018) Update in systemic and targeted therapies in gastrointestinal oncology. Biomedicines 6:34
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Roviello G declares that he has no conflict of interest. Petrioli R declares that he has no conflict of interest. Multari AG declares that he has no conflict of interest. Conca R declares that he has no conflict of interest. Paganini G declares that he has no conflict of interest. Chiariacò G declares that he has no conflict of interest. Aieta M declares that he has no conflict of interest.
Ethical approval
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roviello, G., Petrioli, R., Rosellini, P. et al. The influence of prior ramucirumab treatment on the clinical activity of FOLFIRI as third-line therapy in patients with metastatic gastric Cancer. Invest New Drugs 37, 524–530 (2019). https://doi.org/10.1007/s10637-019-00725-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-019-00725-3