Summary
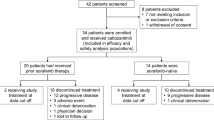
Background The pivotal RESORCE trial showed that regorafenib was effective as second-line therapy for patients with advanced HCC who progressed on first-line sorafenib. Real-world data are needed to assess clinical outcomes and adverse events in the setting of daily practice. Methods Between April 2017 and August 2017, the Named Patient Program (NPP) was activated to provide controlled, pre-approval access of regorafenib in Korea. This analysis is a multicenter retrospective study of patients who received regorafenib under the NPP. Results A total of 49 patients entered into this NPP, and 40 patients received regorafenib in five Korean institutions. All but one patient received regorafenib as second-line therapy after progression on sorafenib, and 36 (90%) and 34 (85%) patients were classified as Child-Pugh A and BCLC stage C, respectively. The response rate was 10% (n = 4). The median progression-free survival (PFS) was 3.7 months (95% CI, 2.5–4.9 months), and the median overall survival (OS) was not reached. The 1 year OS rate was 54.6%. The time-to-progression (TTP) on prior sorafenib was significantly associated with PFS and OS. The most common grade 3–4 toxicities were hand-foot skin reaction (n = 3, 8%), hypertension (n = 2, 5%), and increased aspartate aminotransferase (n = 2, 5%). Conclusion Regorafenib was well-tolerated and effective in patients with advanced HCC who progressed on first-line sorafenib, with efficacy and safety outcomes consistent with those of the previous RESORCE trial. TTP on first-line sorafenib may predict the efficacy of subsequent regorafenib.


Similar content being viewed by others
References
Bruix J, Sherman M, American Association for the Study of Liver Diseases (2011) Management of hepatocellular carcinoma: an update. Hepatology 53:1020–1022. https://doi.org/10.1002/hep.24199
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J, SHARP Investigators Study Group (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390. https://doi.org/10.1056/NEJMoa0708857
Cheng A-L, Kang Y-K, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10:25–34. https://doi.org/10.1016/S1470-2045(08)70285-7
Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D (2011) Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 129:245–255. https://doi.org/10.1002/ijc.25864
Abou-Elkacem L, Arns S, Brix G, Gremse F, Zopf D, Kiessling F, Lederle W (2013) Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther 12:1322–1331. https://doi.org/10.1158/1535-7163.MCT-12-1162
Kissel M, Berndt S, Fiebig L, Kling S, Ji Q, Gu Q, Lang T, Hafner FT, Teufel M, Zopf D (2017) Antitumor effects of regorafenib and sorafenib in preclinical models of hepatocellular carcinoma. Oncotarget 8:107096–107108. https://doi.org/10.18632/oncotarget.22334
Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G (2017) Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389:56–66. https://doi.org/10.1016/S0140-6736(16)32453-9
Abou-Alfa GK, Meyer T, Cheng A-L, el-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen YH, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK (2018) Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 379:54–63. https://doi.org/10.1056/NEJMoa1717002
El-Khoueiry AB, Sangro B, Yau T et al (2017) Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389:2492–2502. https://doi.org/10.1016/S0140-6736(17)31046-2
Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M, Alistar A, Asselah J, Blanc JF, Borbath I, Cannon T, Chung K, Cohn A, Cosgrove DP, Damjanov N, Gupta M, Karino Y, Karwal M, Kaubisch A, Kelley R, van Laethem JL, Larson T, Lee J, Li D, Manhas A, Manji GA, Numata K, Parsons B, Paulson AS, Pinto C, Ramirez R, Ratnam S, Rizell M, Rosmorduc O, Sada Y, Sasaki Y, Stal PI, Strasser S, Trojan J, Vaccaro G, van Vlierberghe H, Weiss A, Weiss KH, Yamashita T (2018) Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 19:940–952. https://doi.org/10.1016/S1470-2045(18)30351-6
Kudo M, Lencioni R, Marrero JA, Venook AP, Bronowicki JP, Chen XP, Dagher L, Furuse J, Geschwind JFH, Ladrón de Guevara L, Papandreou C, Sanyal AJ, Takayama T, Yoon SK, Nakajima K, Lehr R, Heldner S, Ye SL (2016) Regional differences in sorafenib-treated patients with hepatocellular carcinoma: GIDEON observational study. Liver Int 36:1196–1205. https://doi.org/10.1111/liv.13096
Kudo M, Meinhardt G, Bruix J (2017) Regorafenib as second-line treatment of patients with hepatocellular carcinoma (HCC) who progressed on Sorafenib: subgroup analysis of patients from Asia in the phase 3 RESORCE trial. Liver Cancer 6:S8–1. https://doi.org/10.1159/000478029
Yoo C, Kim JE, Lee J-L, Ahn JH, Lee DH, Lee JS, Na S, Kim CS, Hong JH, Hong B, Song C, Ahn H (2010) The efficacy and safety of sunitinib in korean patients with advanced renal cell carcinoma: high incidence of toxicity leads to frequent dose reduction. Jpn J Clin Oncol 40:980–985. https://doi.org/10.1093/jjco/hyq073
Finn RS, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Gerolami R, Caparello C, Cabrera R, Chang C, Sun W, LeBerre MA, Baumhauer A, Meinhardt G, Bruix J (2018) Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: additional analyses from the phase III RESORCE trial. J Hepatol 69:353–358. https://doi.org/10.1016/j.jhep.2018.04.010
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Yoo C, honorarium from Bayer.
Other authors have no conflicts of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was acquired from all patients.
Rights and permissions
About this article
Cite this article
Yoo, C., Park, JW., Kim, Y.J. et al. Multicenter retrospective analysis of the safety and efficacy of regorafenib after progression on sorafenib in Korean patients with hepatocellular carcinoma. Invest New Drugs 37, 567–572 (2019). https://doi.org/10.1007/s10637-018-0707-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0707-5